Chemistry:Ketamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ketalar, others |
| Other names | CI-581; CL-369; CM-52372-2[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Addiction liability | Low–moderate[3] |
| Routes of administration | Any[4][5][6][7] |
| Drug class | NMDA receptor antagonists; General anesthetics; Dissociative hallucinogens; Analgesics; Antidepressants |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | |
| Protein binding | 23 to 47%.[11] |
| Metabolism | Liver, intestine (oral):[6][15][16] |
| Metabolites | |
| Onset of action | |
| Elimination half-life | |
| Duration of action | |
| Excretion | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H16ClNO |
| Molar mass | 237.73 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture:[12]
|
| Melting point | 92[17] °C (198 °F) |
| |
| |
| (verify) | |
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a treatment for depression and pain management.[18] Ketamine is a novel compound that was derived from phencyclidine in 1962 in pursuit of a safer anesthetic with fewer hallucinogenic effects.[19][20]
At anesthetic doses, ketamine induces a state of dissociative anesthesia, a trance-like state providing pain relief, sedation, and amnesia.[21] The distinguishing features of ketamine as anesthesia are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation.[21] At lower, sub-anesthetic doses, ketamine is a promising agent for pain and treatment-resistant depression.[22] As with many antidepressants, the results of a single administration of ketamine wane with time.[23] The long-term effects of repeated use are largely unknown, and are an area of active investigation.[24][25][26]
Liver and urinary toxicity have been reported among regular users of high doses of ketamine for recreational purposes.[27] Ketamine is an NMDA receptor antagonist, accounting for most of its psychoactive effects.[28]
Ketamine was first synthesized in 1962 and approved for use in the United States in 1970.[18] It has been regularly used in veterinary medicine and was extensively used for surgical anesthesia in the Vietnam War.[29] Along with other psychotropic drugs, it is on the World Health Organization's List of Essential Medicines.[30] It is available as a generic medication.[31] When used as a recreational drug, it is found both in crystalline powder and liquid form, and is often referred to by recreational users as "Special K" or simply "K". It is used as a recreational drug for its hallucinogenic and dissociative effects.[32]
Medical uses
Anesthesia
The use of ketamine in anesthesia reflects its characteristics. It is a drug of choice for short-term procedures when muscle relaxation is not required.[33] The effect of ketamine on the respiratory and circulatory systems is different from that of other anesthetics. It suppresses breathing much less than most other available anesthetics.[34] When used at anesthetic doses, ketamine usually stimulates rather than depresses the circulatory system.[35] Protective airway reflexes are preserved[36] and it is sometimes possible to administer ketamine anesthesia without protective measures to the airways.[33] Psychotomimetic effects limit the acceptance of ketamine; however, lamotrigine[37] and nimodipine[38] decrease psychotomimetic effects and can be counteracted also by benzodiazepines or propofol administration.[39] Ketofol is a combination of ketamine and propofol.
Ketamine is frequently used in severely injured people and appears to be safe in this group.[40] It has been widely used for emergency surgery in field conditions in war zones,[41] for example, during the Vietnam War.[42] A 2011 clinical practice guideline supports the use of ketamine as a sedative in emergency medicine, including during physically painful procedures.[21] It is the drug of choice for people in traumatic shock who are at risk of hypotension.[43] Ketamine is unlikely to lower blood pressure, which is dangerous for people with severe head injury;[44] in fact, it can raise blood pressure, often making it useful in treating such injuries.[45][46]
Ketamine is an option in children as the sole anesthetic for minor procedures or as an induction agent followed by neuromuscular blocker and tracheal intubation[41] In particular, children with cyanotic heart disease and neuromuscular disorders are good candidates for ketamine anesthesia.[39]
Due to the bronchodilating properties of ketamine it can be used for anesthesia in people with asthma, chronic obstructive airway disease, and with severe reactive airway disease including active bronchospasm.[41][39][47]
Pain
Ketamine infusions are used for acute pain treatment in emergency departments and in the perioperative period for individuals with refractory pain. The doses are lower than those used for anesthesia; they are usually referred to as sub-anesthetic doses. Adjunctive to morphine or on its own, ketamine reduces morphine use, pain level, nausea, and vomiting after surgery. Ketamine is likely to be most beneficial for surgical patients when severe post-operative pain is expected, and for opioid-tolerant patients.[48][49]
Ketamine is especially useful in the prehospital setting, due to its effectiveness and low risk of respiratory depression.[50] Ketamine has similar efficacy to opioids in a hospital emergency department setting for management of acute pain and for control of procedural pain.[51] It may also prevent opioid-induced hyperalgesia[52][53] and postanesthetic shivering.[54]
For chronic pain, ketamine is used as an intravenous analgesic, particularly if the pain is neuropathic.[20] It has the added benefit of counteracting spinal sensitization or wind-up phenomena experienced with chronic pain.[55] In multiple clinical trials, ketamine infusions delivered short-term pain relief in neuropathic pain diagnoses, pain after traumatic spine injury, fibromyalgia, and complex regional pain syndrome (CRPS).[20] However, the 2018 consensus guidelines on chronic pain concluded that, overall, there is only weak evidence in favor of ketamine use in spinal injury pain, moderate evidence in favor of ketamine for CRPS, and weak or no evidence for ketamine in mixed neuropathic pain, fibromyalgia, and cancer pain. In particular, only for CRPS there is evidence of medium to longer term pain relief.[20]
Depression
Ketamine is a rapid-acting antidepressant,[18] although its effect is transient.[56] Intravenous ketamine infusion in treatment-resistant depression may result in improved mood within 4 hours reaching the peak at 24 hours.[22][24] A single dose of intravenous ketamine has been shown to result in a response rate greater than 60% as early as 4.5 hours after the dose (with a sustained effect after 24 hours) and greater than 40% after 7 days.[57] Although there are only a few pilot studies studying the optimal dose, increasing evidence suggests that 0.5 mg/kg dose injected over 40 minutes gives an optimal outcome.[58] The antidepressant effect of ketamine is diminished at 7 days, and most people relapse within 10 days, although for a significant minority the improvement may last 30 days or more.[24][25][57][59] One of the main challenges with ketamine treatment can be the length of time that the antidepressant effects lasts after finishing a course of treatment. A possible option may be maintenance therapy with ketamine which usually runs twice a week to once in two weeks.[24][25][26] Ketamine may decrease suicidal thoughts for up to three days after the injection.[60]
An enantiomer of ketamine – esketamine commercially sold as Spravato – was approved as an antidepressant by the European Medicines Agency in 2019.[61] Esketamine was approved as a nasal spray for treatment-resistant depression in the United States[62] and elsewhere in 2019 (see Esketamine and Depression). The Canadian Network for Mood and Anxiety Treatments (CANMAT) recommends esketamine as a third-line treatment for depression.[25]
A Cochrane review of randomized controlled trials in adults with unipolar major depressive disorder,[18] found that when compared with placebo, people treated with either ketamine or esketamine experienced reduction or remission of symptoms lasting 1 to 7 days.[63] There were 18.7% (4.1 to 40.4%) more people reporting some benefit and 9.6% (0.2 to 39.4%) more who achieved remission within 24 hours of ketamine treatment. Among people receiving esketamine, 2.1% (2.5 to 24.4%) more encountered some relief at 24 hours and 10.3% (4.5 to 18.2%) more had few or no symptoms. These effects did not persist beyond one week, although higher dropout rate in some studies mean that the duration of benefit remains unclear.[63]
Ketamine may partially improve depressive symptoms[18] among people with bipolar depression, at 24 hours after treatment, but not 3 or more days.[64] Potentially, 10 more people with bipolar depression per 1000 may experience brief improvement, but not cessation of symptoms, one day following treatment. These estimates are based on limited available research.[64]
In February 2022, the US Food and Drug Administration issued an alert to health care professionals concerning compounded nasal spray products containing ketamine intended to treat depression: "There is no FDA-approved ketamine nasal spray product. Compounded drugs are not FDA-approved, which means FDA has not evaluated their safety, effectiveness, or quality prior to marketing."[65]
Near-death experience
Most people who were able to remember their dreams during ketamine anesthesia report near-death experiences (NDE) when the widest possible definition of an NDE is used.[66] Ketamine can reproduce features that commonly have been associated with NDEs.[67] A 2019 large-scale study found that written reports of ketamine experiences had a high degree of similarity to written reports of NDE in comparison to other written reports of drug experiences.[68]
Seizures
Ketamine is used to treat status epilepticus[69] that has not responded to standard treatments, but only case studies and no randomized controlled trials support its use.[70][71]
Asthma
Ketamine has been suggested as a possible therapy for children with severe acute asthma who do not respond to standard treatment.[72] This is due to its bronchodilator effects.[72] A 2012 Cochrane review found there were minimal adverse effects reported, but the limited studies showed no significant benefit.[72]
Contraindications
Some major contraindications for ketamine are:[20][48]
- Severe cardiovascular disease such as unstable angina or poorly controlled hypertension
- Increased intracranial or intraocular pressure (however these remain controversial, with recent studies suggesting otherwise)[48]
- Poorly controlled psychosis
- Severe liver disease such as cirrhosis
- Pregnancy
- Active substance use disorder (for serial ketamine injections)
- Age less than 3 months[9]
Adverse effects
At anesthetic doses, 10–20% of adults and 1–2% of children[9] experience adverse psychiatric reactions that occur during emergence from anesthesia, ranging from dreams and dysphoria to hallucinations and emergence delirium.[73] Psychotomimetic effects decrease adding lamotrigine[37] and nimodipine[38] and can be counteracted by pretreatment with a benzodiazepine or propofol.[73][39] Ketamine anesthesia commonly causes tonic-clonic movements (greater than 10% of people) and rarely hypertonia.[13][73] Vomiting can be expected in 5–15% of the patients; pretreatment with propofol mitigates it as well.[9][73] Laryngospasm occurs only rarely with ketamine. Ketamine, generally, stimulates breathing; however, in the first 2–3 minutes of a high-dose rapid intravenous injection it may cause a transient respiratory depression.[73]
At lower sub-anesthetic doses, psychiatric side effects are prominent. Most people feel strange, spacey, woozy, or a sense of floating, or have visual distortions or numbness. Also very frequent (20–50%) are difficulty speaking, confusion, euphoria, drowsiness, and difficulty concentrating. The symptoms of psychosis such as going into a hole, disappearing, feeling as if melting, experiencing colors, and hallucinations are described by 6–10% of people. Dizziness, blurred vision, dry mouth, hypertension, nausea, increased or decreased body temperature, or feeling flushed are the common (>10%) non-psychiatric side effects. All these adverse effects are most pronounced by the end of the injection, dramatically reduced 40 minutes afterward, and completely disappear within 4 hours after the injection.[74]
Urinary and liver toxicity
Urinary toxicity occurs primarily in people who use large amounts of ketamine routinely, with 20–30% of frequent users having bladder complaints.[20][75] It includes a range of disorders from cystitis to hydronephrosis to kidney failure.[76] The typical symptoms of ketamine-induced cystitis are frequent urination, dysuria, and urinary urgency sometimes accompanied by pain during urination and blood in urine.[77] The damage to the bladder wall has similarities to both interstitial and eosinophilic cystitis. The wall is thickened and the functional bladder capacity is as low as 10–150 mL.[76] Studies indicate that ketamine-induced cystitis is caused by ketamine and its metabolites directly interacting with urothelium, resulting in damage of the epithelial cells of the bladder lining and increased permeability of the urothelial barrier which results in clinical symptoms.[78]
Management of ketamine-induced cystitis involves ketamine cessation as the first step. This is followed by NSAIDs and anticholinergics and, if the response is insufficient, by tramadol. The second line treatments are epithelium-protective agents such as oral pentosan polysulfate or intravesical (intra-bladder) instillation of hyaluronic acid. Intravesical botulinum toxin is also useful.[76]
Liver toxicity of ketamine involves higher doses and repeated administration. In a group of chronic high dose ketamine users, the frequency of liver injury was reported to be about 10%. There are case reports of increased liver enzymes involving ketamine treatment of chronic pain.[76] Chronic ketamine abuse has also been associated with biliary colic,[79] cachexia, gastrointestinal diseases, hepatobiliary disorder, and acute kidney injury.[80]
Dependence and tolerance
Although the incidence of ketamine dependence is unknown, some people who regularly use ketamine develop ketamine dependence. Animal experiments also confirm the risk of misuse.[32] Additionally, the rapid onset of effects following insufflation may increase potential use as a recreational drug. The short duration of effects promotes bingeing. Ketamine tolerance rapidly develops, even with repeated medical use, prompting the use of higher doses. Some daily users reported withdrawal symptoms, primarily anxiety, shaking, sweating, and palpitations, following the attempts to stop.[32] Cognitive deficits as well as increased dissociation and delusion symptoms were observed in frequent recreational users of ketamine.[81]
Interactions
Ketamine potentiates the sedative effects of propofol[82] and midazolam.[83] Naltrexone potentiates psychotomimetic effects of a low dose of ketamine,[84] while lamotrigine[37] and nimodipine[38] decrease them. Clonidine reduces the increase of salivation, heart-rate and blood-pressure during ketamine anesthesia and decreases the incidence of nightmares.[85]
Clinical observations suggest that benzodiazepines may diminish the antidepressant effects of ketamine.[86] It appears most conventional antidepressants can be safely combined with ketamine.[86]
Pharmacology
Pharmacodynamics
Mechanism of action
Pore blocking of the NMDA receptor is responsible for the anesthetic, analgesic, and psychotomimetic effects of ketamine.[28][87] Blocking of the NMDA receptor results in analgesia by preventing central sensitization in dorsal horn neurons; in other words, ketamine's actions interfere with pain transmission in the spinal cord.[13]
The mechanism of action of ketamine in alleviating depression is not well understood, and is an area of active investigation. Possible mechanisms include direct action on the NMDA receptor, downstream effects on regulators such as BDNF and mTOR, and effects of ketamine's metabolites such as hydroxynorketamine.[88] It is not clear whether NMDA receptor is solely responsible for this action or interactions with other receptors are also necessary. It is also not clear whether ketamine alone is sufficient for the antidepressive action or its metabolites also are important.[28][88][89] In any case, it has been elucidated that acute blockade of NMDA receptors in the brain results in an increase in the release of glutamate, which leads to an activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA receptors), which in turn modulate a variety of downstream signaling pathways to influence neurotransmission in the limbic system and mediate antidepressant effects of NMDA receptor antagonists like ketamine.[57][88][90] Such downstream actions of this activation of AMPA receptors include upregulation of brain-derived neurotrophic factor (BDNF) and activation of its signaling receptor tropomyosin receptor kinase B (TrkB), activation of the mammalian target of rapamycin (mTOR) pathway, deactivation of glycogen synthase kinase 3 (GSK-3), and inhibition of the phosphorylation of the eukaryotic elongation factor 2 (eEF2) kinase.[57][88][91][92] In addition to blockade of the NMDA receptor, the active metabolite of ketamine hydroxynorketamine, which does not interact importantly with the NMDA receptor, but nonetheless indirectly activates AMPA receptors similarly, may also or alternatively be involved in the rapid-onset antidepressant effects of ketamine.[88][89] Recent research has elucidated that an acute inhibition of the lateral habenula, a part of the brain in the limbic system that has been referred to as the "anti-reward center" (projecting to and inhibiting the mesolimbic reward pathway and modulating other limbic areas), may be involved in the antidepressant effects of ketamine.[88][93][94]
Ketamine is a mixture of equal amounts of two enantiomers: esketamine and arketamine. Esketamine is a more potent NMDA receptor pore blocker and dissociative hallucinogen than arketamine.[10] Because of the hypothesis that NMDA receptor antagonism underlies the antidepressant effects of ketamine, esketamine was developed as an antidepressant.[10] However, multiple other NMDA receptor antagonists, including memantine, lanicemine, rislenemdaz, rapastinel, and 4-chlorokynurenine, thus far have failed to demonstrate sufficient effectiveness for depression.[10][95] Furthermore, animal research indicates that arketamine, the enantiomer with a weaker NMDA receptor antagonism, as well as (2R,6R)-hydroxynorketamine, the metabolite with negligible affinity for the NMDA receptor, but a potent alpha-7 nicotinic receptor antagonist may have antidepressive action.[10][96] It is now argued that NMDA receptor antagonism may not be primarily responsible for the antidepressant effects of ketamine.[10][97][95]
Molecular targets
| Site | Value (μM) | Type | Action | Species | Ref |
|---|---|---|---|---|---|
| MOR2 | 12.1 | Ki | Antagonist | Human | [98] |
| KOR | 28 25 |
Ki Ki |
Antagonist Agonist |
Human | [99] [100] |
| σ2 | 26 | Ki | ND | Rat | [101] |
| D2 | 0.5 >10 |
Ki Ki |
Agonist ND |
Human | [102] [103][104][105] |
| M1 | 45 | Ki | ND | Human | [106] |
| α2β4 | 29 | IC50 | Antagonist | Human | [107] |
| α3β2 | 50 | IC50 | Antagonist | Human | [107] |
| α3β4 | 9.5 | IC50 | Antagonist | Human | [107] |
| α4β2 | 72 | IC50 | Antagonist | Human | [107] |
| α4β4 | 18 | IC50 | Antagonist | Human | [107] |
| α7 | 3.1 | IC50 | Antagonist | Rat | [96] |
| DAT | 63 | Ki | Inhibitor | Rat | [108] |
| TRPV1 | 1-100 | Ki | Agonist | Rat | [109] |
| The smaller the value, the stronger the interaction with the site. | |||||
Ketamine principally acts as a pore blocker of the NMDA receptor, an ionotropic glutamate receptor.[110] The S-(+) and R-(–) stereoisomers of ketamine bind to the dizocilpine site of the NMDA receptor with different affinities, the former showing approximately 3- to 4-fold greater affinity for the receptor than the latter. As a result, the S isomer is a more potent anesthetic and analgesic than its R counterpart.[111]
Ketamine may interact with and inhibit the NMDAR via another allosteric site on the receptor.[112]
With a couple of exceptions, ketamine actions at other receptors are far weaker than ketamine's antagonism of the NMDA receptor (see the activity table to the right).[6][113]
Although ketamine is a very weak ligand of the monoamine transporters (Ki > 60 μM), it has been suggested that it may interact with allosteric sites on the monoamine transporters to produce monoamine reuptake inhibition.[103] However, no functional inhibition (IC50) of the human monoamine transporters has been observed with ketamine or its metabolites at concentrations of up to 10,000 nM.[104][110] Moreover, animal studies and at least three human case reports have found no interaction between ketamine and the monoamine oxidase inhibitor (MAOI) tranylcypromine, which is of importance as the combination of a monoamine reuptake inhibitor with an MAOI can produce severe toxicity such as serotonin syndrome or hypertensive crisis.[114][115] Collectively, these findings shed doubt on the involvement of monoamine reuptake inhibition in the effects of ketamine in humans.[114][110][104][115] Ketamine has been found to increase dopaminergic neurotransmission in the brain, but instead of being due to dopamine reuptake inhibition, this may be via indirect/downstream mechanisms, namely through antagonism of the NMDA receptor.[110][104]
Whether ketamine is an agonist of D2 receptors is controversial. Early research by the Philip Seeman group found ketamine to be a D2 partial agonist with the potency similar to that of its NMDA receptor antagonism.[102][116][117] However, later studies by different researchers found the affinity of ketamine of >10 μM for the regular human and rat D2 receptors,[103][104][105] Moreover, whereas D2 receptor agonists such as bromocriptine are able to rapidly and powerfully suppress prolactin secretion,[118] subanesthetic doses of ketamine have not been found to do this in humans and in fact, have been found to dose-dependently increase prolactin levels.[119][120] Imaging studies have shown mixed results on inhibition of striatal [11C] raclopride binding by ketamine in humans, with some studies finding a significant decrease and others finding no such effect.[121] However, changes in [11C] raclopride binding may be due to changes in dopamine concentrations induced by ketamine rather than binding of ketamine to the D2 receptor.[121]
Relationships between levels and effects
Dissociation and psychotomimetic effects are reported in people treated with ketamine at plasma concentrations of approximately 100 to 250 ng/mL (0.42–1.1 μM).[28] The typical intravenous antidepressant dosage of ketamine used to treat depression is low and results in maximal plasma concentrations of 70 to 200 ng/mL (0.29–0.84 μM).[56] At similar plasma concentrations (70 to 160 ng/mL; 0.29–0.67 μM) it also shows analgesic effects.[56] In 1–5 minutes after inducing anesthesia by a rapid intravenous injection of ketamine, its plasma concentration reaches as high as 60–110 μM.[122][123] When the anesthesia was maintained using nitrous oxide together with continuous injection of ketamine, the ketamine concentration stabilized at approximately 9.3 μM.[122] In an experiment with purely ketamine anesthesia, people began to awaken once the plasma level of ketamine decreased to about 2,600 ng/mL (11 μM) and became oriented in place and time when the level was down to 1,000 ng/mL (4 μM).[124] In a single-case study, the concentration of ketamine in cerebrospinal fluid, a proxy for the brain concentration, during anesthesia varied between 2.8 and 6.5 μM and was approximately 40% lower than in plasma.[125]
Pharmacokinetics
Ketamine can be absorbed by many different routes due to both its water and lipid solubility. Intravenous ketamine bioavailability is 100% by definition, intramuscular injection bioavailability is slightly lower at 93%,[6] and epidural bioavailability is 77%.[8] Subcutaneous bioavailability has never been measured, but is presumed to be high.[126] Among the less invasive routes, the intranasal route has the highest bioavailability (45–50%)[6][9] and oral – the lowest (16–20%).[6][9] Sublingual and rectal bioavailabilities are intermediate at approximately 25–50%.[6][10][9]
After absorption ketamine is rapidly distributed into the brain and other tissues.[87] The plasma protein binding of ketamine is variable at 23–47%.[11]
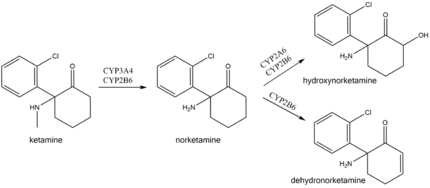
In the body ketamine undergoes extensive metabolism. It is biotransformed by CYP3A4 and CYP2B6 isoenzymes into norketamine, which, in turn, is converted by CYP2A6 and CYP2B6 into hydroxynorketamine and dehydronorketamine.[28] Low oral bioavailability of ketamine is due to the first-pass effect and, possibly, ketamine intestinal metabolism by CYP3A4.[16] As a result, norketamine plasma levels are several-fold higher than ketamine following oral administration, and norketamine may play a role in anesthetic and analgesic action of oral ketamine.[6][16] This also explains why oral ketamine levels are independent of CYP2B6 activity, unlike subcutaneous ketamine levels.[16][127]
After an intravenous injection of tritium-labelled ketamine, 91% of the radioactivity is recovered from urine and 3% from the feces.[14] The medication is excreted mostly in the form of metabolites, with only 2% remaining unchanged. Conjugated hydroxylated derivatives of ketamine (80%) followed by dehydronorketamine (16%) are the most prevalent metabolites detected in urine.[29]
Chemistry
Synthesis
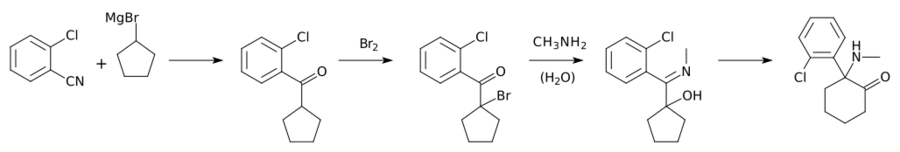
2-chlorobenzonitrile is reacted with the Grignard reagent cyclopentylmagnesium bromide to give (2-chlorophenyl)(cyclopentyl)methanone. This is then brominated using bromine to form the corresponding bromoketone, which is then reacted with methylamine in an aqueous solution to form the methylimino derivative, 1-(2-Chloro-N-methylbenzimidoyl)cyclopentanol, with hydrolysis of the tertiary bromine atom. This final intermediate is then heated in decalin or another suitable high-boiling solvent, upon which an Alpha-ketol rearrangement occurs resulting in a ring-expansion, and the formation of racemic ketamine.
Structure
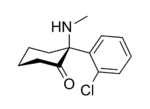
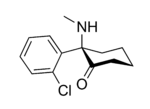
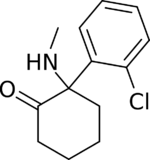
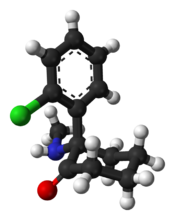
In chemical structure, ketamine is an arylcyclohexylamine derivative. Ketamine is a chiral compound. The more active enantiomer, esketamine (S-ketamine), is also available for medical use under the brand name Ketanest S,[129] while the less active enantiomer, arketamine (R-ketamine), has never been marketed as an enantiopure drug for clinical use. While S-ketamine is more effective as an analgesic and anesthetic through NMDA receptor antagonism, R-ketamine produces longer-lasting effects as an antidepressant.[18]
The optical rotation of a given enantiomer of ketamine can vary between its salts and free base form. The free base form of (S)‑ketamine exhibits dextrorotation and is therefore labelled (S)‑(+)‑ketamine. However, its hydrochloride salt shows levorotation and is thus labelled (S)‑(−)‑ketamine hydrochloride.[130]
Detection
Ketamine may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized people, provide evidence in an impaired driving arrest, or to assist in a medicolegal death investigation. Blood or plasma ketamine concentrations are usually in a range of 0.5–5.0 mg/L in persons receiving the drug therapeutically (during general anesthesia), 1–2 mg/L in those arrested for impaired driving and 3–20 mg/L in victims of acute fatal overdosage. Urine is often the preferred specimen for routine drug use monitoring purposes. The presence of norketamine, a pharmacologically active metabolite, is useful for confirmation of ketamine ingestion.[131][132][133]
History
Ketamine was first synthesized in 1962 by Calvin L. Stevens,[18] a professor of chemistry at Wayne State University and a Parke-Davis consultant. It was known by the developmental code name CI-581.[18] After promising preclinical research in animals, ketamine was tested in human prisoners in 1964.[29] These investigations demonstrated ketamine's short duration of action and reduced behavioral toxicity made it a favorable choice over phencyclidine (PCP) as an anesthetic.[134] The researchers wanted to call the state of ketamine anesthesia "dreaming", but Parke-Davis did not approve of the name. Hearing about this problem and about the "disconnected" appearance of treated people, Mrs. Edward F. Domino,[135] the wife of one of the pharmacologists working on ketamine, suggested "dissociative anesthesia".[29] Following FDA approval in 1970, ketamine anesthesia was first given to American soldiers during the Vietnam War.[136]
The discovery of antidepressive action of ketamine in 2000[137] has been described as the single most important advance in the treatment of depression in more than 50 years.[59][10] It has sparked interest in NMDA receptor antagonists for depression,[138] and has shifted the direction of antidepressant research and development.[139]
Society and culture
Legal status
While ketamine is marketed legally in many countries worldwide,[140] it is also a controlled substance in many countries.[6]
- In Australia , ketamine is listed as a schedule 8 controlled drug under the Poisons Standard (October 2015).[141]
- In Canada , ketamine is classified as a Schedule I narcotic, since 2005.[142]
- In December 2013, the government of India, in response to rising recreational use and the use of ketamine as a date rape drug, has added it to Schedule X of the Drug and Cosmetics Act requiring a special license for sale and maintenance of records of all sales for two years.[143][144]
- In the United Kingdom , it became labeled a Class B drug on 12 February 2014.[145][146]
- The increase in recreational use prompted ketamine to be placed in Schedule III of the United States Controlled Substances Act in August 1999.[147]
Recreational use
At sub-anesthetic doses ketamine produces a dissociative state, characterised by a sense of detachment from one's physical body and the external world that is known as depersonalization and derealization.[148] At sufficiently high doses, users may experience what is called the "K-hole", a state of dissociation with visual and auditory hallucination.[149] John C. Lilly, Marcia Moore, D. M. Turner, and David Woodard (among others) have written extensively about their own entheogenic and psychonautic experiences with ketamine.[150] Turner died prematurely due to drowning during presumed unsupervised ketamine use.[151] In 2006, the Russian edition of Adam Parfrey's Apocalypse Culture II was banned and destroyed by authorities owing to its inclusion of an essay by Woodard about the entheogenic use of, and psychonautic experiences with, ketamine.[152]:288–295 Recreational ketamine use has been implicated in deaths globally, with more than 90 deaths in England and Wales in the years of 2005–2013.[153] They include accidental poisonings, drownings, traffic accidents, and suicides.[153] The majority of deaths were among young people.[154] Several months after being found dead in his hot tub, actor Matthew Perry's October, 2023 apparent drowning death was revealed to have been caused by a ketamine overdose, and while other factors were present, the acute effects of ketamine were ruled to be the primary cause of death.[155] Because of its ability to cause confusion and amnesia, ketamine has been used for date rape.[156][136]
Research
Ketamine is under investigation for its potential in treating treatment-resistant depression.[157][158][159] Ketamine is a known psychoplastogen, which refers to a compound capable of promoting rapid and sustained neuroplasticity.[160]
Ketamine has shown anthelmintic activity in rats, with an effect comparable to ivermectin and albendazole at extremely high concentrations.[161]
Veterinary medicine
In veterinary anesthesia, ketamine is often used for its anesthetic and analgesic effects on cats,[162] dogs,[163] rabbits, rats, and other small animals.[164][165] It is frequently used in induction and anesthetic maintenance in horses. It is an important part of the "rodent cocktail", a mixture of drugs used for anesthetising rodents.[166] Veterinarians often use ketamine with sedative drugs to produce balanced anesthesia and analgesia, and as a constant-rate infusion to help prevent pain wind-up. Ketamine is also used to manage pain among large animals. It is the primary intravenous anesthetic agent used in equine surgery, often in conjunction with detomidine and thiopental, or sometimes guaifenesin.[167]
Ketamine appears not to produce sedation or anesthesia in snails. Instead, it appears to have an excitatory effect.[168]
References
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 159–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA159.
- ↑ "Ketamine (Ketalar) Use During Pregnancy". 22 November 2019. https://www.drugs.com/pregnancy/ketamine.html.
- ↑ "Drug Scheduling". US DEA. https://www.dea.gov/drug-information/drug-scheduling. Ketamine is listed in Schedule III.
- ↑ "Ketamine as an adjuvant to opioids for cancer pain". The Cochrane Database of Systematic Reviews 6 (9): CD003351. June 2017. doi:10.1002/14651858.CD003351.pub3. PMID 28657160. PMC 6481583. http://opus.bath.ac.uk/57535/1/Published_Version.pdf.
- ↑ "Perioperative Ketamine Administration for Thoracotomy Pain". Pain Physician 20 (3): 173–184. March 2017. PMID 28339431.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 Ketamine for Treatment-Resistant Depression: The First Decade of Progress. Springer. 25 November 2016. pp. 8–10, 14–22. ISBN 978-3-319-42925-0. https://books.google.com/books?id=QDOgDQAAQBAJ&pg=PA22.
- ↑ "Ketamine Hydrochloride: Martindale: The Complete Drug Reference". London, UK: Pharmaceutical Press. 9 January 2017. https://www.medicinescomplete.com/mc/martindale/current/ms-3114-h.htm.
- ↑ 8.0 8.1 Toxicological Aspects of Drug-Facilitated Crimes. Elsevier Science. 22 March 2014. pp. 87–. ISBN 978-0-12-416969-2. https://books.google.com/books?id=YgnUAgAAQBAJ&pg=PA87.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 "Ketamine: use in anesthesia". CNS Neurosci Ther 19 (6): 381–9. June 2013. doi:10.1111/cns.12072. PMID 23521979.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences 73 (10): 613–627. October 2019. doi:10.1111/pcn.12902. PMID 31215725.
- ↑ 11.0 11.1 "The binding of ketamine to plasma proteins: emphasis on human plasma". Eur J Clin Pharmacol 24 (6): 825–31. 1983. doi:10.1007/BF00607095. PMID 6884418.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 "Ketamine". Modern Anesthetics. Handbook of Experimental Pharmacology. 182. 2008. pp. 313–33. doi:10.1007/978-3-540-74806-9_15. ISBN 978-3-540-72813-9.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 "Ketamine*". Journal of Pain and Symptom Management 41 (3): 640–9. March 2011. doi:10.1016/j.jpainsymman.2011.01.001. PMID 21419322. http://www.jpsmjournal.com/article/S0885-3924%2811%2900046-7/fulltext. Retrieved 28 July 2014.
- ↑ 14.0 14.1 14.2 "Biotransformation and disposition of ketamine". Int Anesthesiol Clin 12 (2): 157–77. 1974. doi:10.1097/00004311-197412020-00018. PMID 4603048.
- ↑ "Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes". Drug Metabolism and Disposition 30 (7): 853–8. July 2002. doi:10.1124/dmd.30.7.853. PMID 12065445.
- ↑ 16.0 16.1 16.2 16.3 "Role of Cytochrome P4502B6 Polymorphisms in Ketamine Metabolism and Clearance". Anesthesiology 125 (6): 1103–1112. December 2016. doi:10.1097/ALN.0000000000001392. PMID 27763887.
- ↑ "Ketamine". Analytical Profiles of Drug Substances. 6. Academic Press. 1977. pp. 297–322. doi:10.1016/S0099-5428(08)60347-0. ISBN 9780122608063.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 "Ketamine as a therapeutic agent in major depressive disorder and posttraumatic stress disorder: Potential medicinal and deleterious effects" (in en). Ibrain 9 (1): 90–101. March 2023. doi:10.1002/ibra.12094. ISSN 2769-2795. PMID 37786516.
- ↑ "Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy". Clinical Pharmacokinetics 55 (9): 1059–77. September 2016. doi:10.1007/s40262-016-0383-6. PMID 27028535.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 "Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists". Reg Anesth Pain Med 43 (5): 521–546. July 2018. doi:10.1097/AAP.0000000000000808. PMID 29870458.
- ↑ 21.0 21.1 21.2 "Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update". Annals of Emergency Medicine 57 (5): 449–461. May 2011. doi:10.1016/j.annemergmed.2010.11.030. PMID 21256625.
- ↑ 22.0 22.1 "An update on ketamine and its two enantiomers as rapid-acting antidepressants". Expert Review of Neurotherapeutics 19 (1): 83–92. January 2019. doi:10.1080/14737175.2019.1554434. PMID 30513009.
- ↑ "Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression". ACS Chemical Neuroscience 11 (6): 864–871. March 2020. doi:10.1021/acschemneuro.9b00493. PMID 32133835.
- ↑ 24.0 24.1 24.2 24.3 "A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019". J Affect Disord 277: 831–841. December 2020. doi:10.1016/j.jad.2020.09.007. PMID 33065824.
- ↑ 25.0 25.1 25.2 25.3 "The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommandations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L'humeur Et De L'anxiété (Canmat) Concernant L'utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur". Can J Psychiatry 66 (2): 113–125. November 2020. doi:10.1177/0706743720970860. PMID 33174760.
- ↑ 26.0 26.1 "Next-Step Treatment Considerations for Patients With Treatment-Resistant Depression That Responds to Low-Dose Intravenous Ketamine". Focus (Am Psychiatr Publ) 18 (2): 181–192. April 2020. doi:10.1176/appi.focus.20190048. PMID 33162856.
- ↑ "Ketamine toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. April 2022. https://www.ncbi.nlm.nih.gov/books/NBK541087/. Retrieved 18 August 2022.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 "Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms". Pharmacol Rev 70 (3): 621–660. July 2018. doi:10.1124/pr.117.015198. PMID 29945898.
- ↑ 29.0 29.1 29.2 29.3 "Taming the ketamine tiger. 1965". Anesthesiology 113 (3): 678–84. September 2010. doi:10.1097/ALN.0b013e3181ed09a2. PMID 20693870.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Ketamine Injection". https://www.drugs.com/pro/ketamine-injection.html.
- ↑ 32.0 32.1 32.2 "Ketamine use: a review". Addiction 107 (1): 27–38. January 2012. doi:10.1111/j.1360-0443.2011.03576.x. PMID 21777321.
- ↑ 33.0 33.1 "Ketamine". StatPearls. StatPearls Publishing. 2020. http://www.ncbi.nlm.nih.gov/books/NBK470357/. Retrieved 5 March 2020.
- ↑ "Use of ketamine in severe status asthmaticus in intensive care unit". Iranian Journal of Allergy, Asthma, and Immunology 2 (4): 175–80. December 2003. PMID 17301376. http://ijaai.tums.ac.ir/index.php/ijaai/article/view/52/52.
- ↑ "[S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation]" (in DE). Der Anaesthesist 46 (12): 1081–7. December 1997. doi:10.1007/s001010050510. PMID 9451493.
- ↑ "A review of the use of adjunctive therapies in severe acute asthma exacerbation in critically ill children". Expert Review of Respiratory Medicine 8 (4): 423–41. August 2014. doi:10.1586/17476348.2014.915752. PMID 24993063.
- ↑ 37.0 37.1 37.2 "Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists". Arch Gen Psychiatry 57 (3): 270–6. March 2000. doi:10.1001/archpsyc.57.3.270. PMID 10711913.
- ↑ 38.0 38.1 38.2 "Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists". Neuropsychopharmacology 25 (6): 936–47. December 2001. doi:10.1016/S0893-133X(01)00346-3. PMID 11750186.
- ↑ 39.0 39.1 39.2 39.3 "Ketamine: a versatile tool for anesthesia and analgesia". Current Opinion in Anesthesiology 33 (5): 633–638. October 2020. doi:10.1097/ACO.0000000000000916. PMID 32826629.
- ↑ "The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review". Annals of Emergency Medicine 65 (1): 43–51.e2. January 2015. doi:10.1016/j.annemergmed.2014.06.018. PMID 25064742.
- ↑ 41.0 41.1 41.2 "Ketamine: Current applications in anesthesia, pain, and critical care". Anesthesia: Essays and Researches 8 (3): 283–90. September 2014. doi:10.4103/0259-1162.143110. PMID 25886322.
- ↑ "History of anaesthesia: The ketamine story - past, present and future". Eur J Anaesthesiol 34 (9): 571–575. September 2017. doi:10.1097/EJA.0000000000000638. PMID 28731926.
- ↑ "Intubation, Hypotension and Shock". Critical Care Compendium. 7 August 2013. http://lifeinthefastlane.com/education/ccc/rapid-sequence-induction-of-the-shock-patient/.[unreliable medical source?]
- ↑ "Hypotension, hypoxia, and head injury: frequency, duration, and consequences". Archives of Surgery 136 (10): 1118–23. October 2001. doi:10.1001/archsurg.136.10.1118. PMID 11585502.
- ↑ "Intravenous ketamine for prevention of severe hypotension during spinal anaesthesia". Acta Anaesthesiologica Scandinavica 35 (8): 755–7. November 1991. doi:10.1111/j.1399-6576.1991.tb03385.x. PMID 1763596.
- ↑ "The cardiovascular effects of ketamine in hypotensive states". Canadian Anaesthetists' Society Journal 22 (3): 339–48. May 1975. doi:10.1007/BF03004843. PMID 1139377.
- ↑ "Ketamine in status asthmaticus: A review". Indian Journal of Critical Care Medicine 17 (3): 154–61. May 2013. doi:10.4103/0972-5229.117048. PMID 24082612.
- ↑ 48.0 48.1 48.2 "Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists". Reg Anesth Pain Med 43 (5): 456–466. July 2018. doi:10.1097/AAP.0000000000000806. PMID 29870457.
- ↑ "The use of subdissociative-dose ketamine for acute pain in the emergency department". Academic Emergency Medicine 22 (3): 251–7. March 2015. doi:10.1111/acem.12604. PMID 25716117.
- ↑ "Ketamine: a unique drug with several potential uses in the prehospital setting". Journal of Paramedic Practice 3 (10): 552–556. 2011. doi:10.12968/jpar.2011.3.10.552.
- ↑ "A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department". Academic Emergency Medicine 25 (10): 1086–1097. October 2018. doi:10.1111/acem.13502. PMID 30019434.
- ↑ "Role of ketamine in acute postoperative pain management: a narrative review". BioMed Research International 2015: 749837. 2015. doi:10.1155/2015/749837. PMID 26495312.
- ↑ "A comprehensive review of opioid-induced hyperalgesia". Pain Physician 14 (2): 145–61. 2011. doi:10.36076/ppj.2011/14/145. PMID 21412369.
- ↑ "Efficacy and safety of prophylactic use of ketamine for prevention of postanesthetic shivering: a systematic review and meta analysis". BMC Anesthesiology 19 (1): 245. December 2019. doi:10.1186/s12871-019-0910-8. PMID 31888509.
- ↑ "Ketamine and postoperative pain—a quantitative systematic review of randomised trials". Pain 113 (1–2): 61–70. January 2005. doi:10.1016/j.pain.2004.09.036. PMID 15621365.
- ↑ 56.0 56.1 56.2 "A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders". JAMA Psychiatry 74 (4): 399–405. April 2017. doi:10.1001/jamapsychiatry.2017.0080. PMID 28249076.
- ↑ 57.0 57.1 57.2 57.3 "Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review". CNS Drugs 32 (5): 411–420. May 2018. doi:10.1007/s40263-018-0519-3. PMID 29736744.
- ↑ "Ketamine: A Review for Clinicians". Focus (American Psychiatric Association Publishing) 16 (3): 243–250. July 2018. doi:10.1176/appi.focus.20180012. PMID 31975918.
- ↑ 59.0 59.1 "Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight". The Lancet. Psychiatry 4 (5): 419–426. May 2017. doi:10.1016/S2215-0366(17)30102-5. PMID 28395988. http://discovery.ucl.ac.uk/1552865/. Retrieved 10 September 2018.
- ↑ "Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials". Aust N Z J Psychiatry 54 (1): 29–45. January 2020. doi:10.1177/0004867419883341. PMID 31729893. https://ora.ox.ac.uk/objects/uuid:25219d6c-5c8f-4842-91d9-41a9fd7fb1bd. Retrieved 18 January 2021.
- ↑ "Spravato (esketamine)". European Medicines Agency. 8 July 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/spravato. Retrieved 20 July 2022.
- ↑ "FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor's office or clinic". US Food and Drug Administration. 5 March 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified.
- ↑ 63.0 63.1 "Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder". The Cochrane Database of Systematic Reviews 9 (11): CD011612. September 2021. doi:10.1002/14651858.CD011612.pub3. PMID 34510411.
- ↑ 64.0 64.1 "Ketamine and other glutamate receptor modulators for depression in adults with bipolar disorder". The Cochrane Database of Systematic Reviews 2021 (10): CD011611. October 2021. doi:10.1002/14651858.CD011611.pub3. PMID 34623633.
- ↑ "FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray". US Food and Drug Administration. 16 February 2022. https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-professionals-potential-risks-associated-compounded-ketamine-nasal-spray.
- ↑ Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. 2001. p. 122. ISBN 978-0-9660019-3-8.
- ↑ "Semiology and Mechanisms of Near-Death Experiences". Current Neurology and Neuroscience Reports 19 (9): 62. July 2019. doi:10.1007/s11910-019-0983-2. PMID 31352520.
- ↑ "Neurochemical models of near-death experiences: A large-scale study based on the semantic similarity of written reports". Consciousness and Cognition 69: 52–69. March 2019. doi:10.1016/j.concog.2019.01.011. PMID 30711788.
- ↑ "Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy". Biomedicines 9 (5): 470. April 2021. doi:10.3390/biomedicines9050470. PMID 33923061.
- ↑ "Consensus Protocol for the Treatment of Super-Refractory Status Epilepticus". Acta Médica Portuguesa 31 (10): 598–605. October 2018. doi:10.20344/amp.9679. PMID 30387431. https://www.actamedicaportuguesa.com/revista/index.php/amp/article/view/9679. Retrieved 11 February 2020.
- ↑ "Ketamine for Refractory Status Epilepticus: A Systematic Review". CNS Drugs 32 (11): 997–1009. November 2018. doi:10.1007/s40263-018-0569-6. PMID 30232735.
- ↑ 72.0 72.1 72.2 "Ketamine for management of acute exacerbations of asthma in children". The Cochrane Database of Systematic Reviews 11 (11): CD009293. November 2012. doi:10.1002/14651858.CD009293.pub2. PMID 23152273.
- ↑ 73.0 73.1 73.2 73.3 73.4 "Adverse events associated with ketamine for procedural sedation in adults". The American Journal of Emergency Medicine 26 (9): 985–1028. November 2008. doi:10.1016/j.ajem.2007.12.005. PMID 19091264. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0026626/.
- ↑ "Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression". J Affect Disord 263: 568–575. February 2020. doi:10.1016/j.jad.2019.11.028. PMID 31791675.
- ↑ "Ketamine-induced urologic insult (KIUI)". Pain Physician 13 (6): E343–6. 2010. doi:10.36076/ppj.2010/13/E343. PMID 21102971.
- ↑ 76.0 76.1 76.2 76.3 "What urologists need to know about ketamine-induced uropathy: A systematic review". Neurourol Urodyn 39 (4): 1049–1062. April 2020. doi:10.1002/nau.24341. PMID 32212278.
- ↑ "Ketamine-induced vesicopathy: a literature review". International Journal of Clinical Practice 65 (1): 27–30. January 2011. doi:10.1111/j.1742-1241.2010.02502.x. PMID 21155941. https://hal.archives-ouvertes.fr/hal-00600043. Retrieved 10 September 2018.
- ↑ "Changes to the bladder epithelial barrier are associated with ketamine-induced cystitis". Experimental and Therapeutic Medicine 14 (4): 2757–2762. 20 January 2017. doi:10.3892/etm.2017.4913. PMID 28966667.
- ↑ "Chronic biliary colic associated with ketamine abuse". International Medical Case Reports Journal 9: 135–137. 2 June 2016. doi:10.2147/IMCRJ.S100648. PMID 27330331.
- ↑ "Multiorgan Dysfunction Related to Chronic Ketamine Abuse". Baylor University Medical Center Proceedings 27 (3): 223–225. 11 December 2017. doi:10.1080/08998280.2014.11929117. PMID 24982568.
- ↑ "Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study". Addiction 105 (1): 121–33. January 2010. doi:10.1111/j.1360-0443.2009.02761.x. PMID 19919593.
- ↑ "Additive interactions between propofol and ketamine when used for anesthesia induction in female patients". Anesthesiology 82 (3): 641–8. March 1995. doi:10.1097/00000542-199503000-00005. PMID 7879932.
- ↑ "Hypnotic and anesthetic interactions between ketamine and midazolam in female patients". Anesthesiology 79 (6): 1227–32. December 1993. doi:10.1097/00000542-199312000-00013. PMID 8267198.
- ↑ "Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism". Neuropsychopharmacology 31 (8): 1793–800. August 2006. doi:10.1038/sj.npp.1300994. PMID 16395307.
- ↑ "Effects of oral clonidine premedication on side effects of intravenous ketamine anesthesia: a randomized, double-blind, placebo-controlled study". J Clin Anesth 12 (1): 19–24. February 2000. doi:10.1016/s0952-8180(99)00131-2. PMID 10773503.
- ↑ 86.0 86.1 "Ketamine for Depression, 5: Potential Pharmacokinetic and Pharmacodynamic Drug Interactions". The Journal of Clinical Psychiatry 78 (7): e858–e861. July 2017. doi:10.4088/JCP.17f11802. PMID 28858450.
- ↑ 87.0 87.1 "Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy". Clin Pharmacokinet 55 (9): 1059–77. September 2016. doi:10.1007/s40262-016-0383-6. PMID 27028535.
- ↑ 88.0 88.1 88.2 88.3 88.4 88.5 "Mechanisms of ketamine action as an antidepressant". Molecular Psychiatry 23 (4): 801–811. April 2018. doi:10.1038/mp.2017.255. PMID 29532791.
- ↑ 89.0 89.1 "Convergent Mechanisms Underlying Rapid Antidepressant Action". CNS Drugs 32 (3): 197–227. March 2018. doi:10.1007/s40263-018-0492-x. PMID 29516301.
- ↑ "Glutamatergic Signaling Drives Ketamine-Mediated Response in Depression: Evidence from Dynamic Causal Modeling". The International Journal of Neuropsychopharmacology 21 (8): 740–747. August 2018. doi:10.1093/ijnp/pyy041. PMID 29668918.
- ↑ "BDNF - a key transducer of antidepressant effects". Neuropharmacology 102: 72–79. March 2016. doi:10.1016/j.neuropharm.2015.10.034. PMID 26519901.
- ↑ "Brain-derived neurotrophic factor in mood disorders and antidepressant treatments". Neurobiology of Disease 97 (Pt B): 119–126. January 2017. doi:10.1016/j.nbd.2016.07.010. PMID 27425886.
- ↑ "Overcoming Depression by Inhibition of Neural Burst Firing". Neuron 98 (5): 878–879. June 2018. doi:10.1016/j.neuron.2018.05.032. PMID 29879390.
- ↑ "Ketamine blocks bursting in the lateral habenula to rapidly relieve depression". Nature 554 (7692): 317–322. February 2018. doi:10.1038/nature25509. PMID 29446381. Bibcode: 2018Natur.554..317Y.
- ↑ 95.0 95.1 "The development of glutamate-based antidepressants is taking longer than expected". Drug Discovery Today 23 (10): 1689–1692. October 2018. doi:10.1016/j.drudis.2018.02.006. PMID 29501913.
- ↑ 96.0 96.1 "Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors". Eur J Pharmacol 698 (1–3): 228–34. January 2013. doi:10.1016/j.ejphar.2012.11.023. PMID 23183107.
- ↑ "Arketamine – Jiangsu Hengrui Medicine – AdisInsight". https://adisinsight.springer.com/drugs/800056158.
- ↑ "Interaction of ketamine with mu2 opioid receptors in SH-SY5Y human neuroblastoma cells". Journal of Anesthesia 13 (2): 107–9. 1999. doi:10.1007/s005400050035. PMID 14530949.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9915326 - ↑ "Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats". Psychopharmacology (Berl) 210 (2): 263–74. June 2010. doi:10.1007/s00213-010-1834-7. PMID 20358363.
- ↑ "Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo". Eur Neuropsychopharmacol 22 (4): 308–17. April 2012. doi:10.1016/j.euroneuro.2011.08.002. PMID 21911285.
- ↑ 102.0 102.1 "NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia". Molecular Psychiatry 7 (8): 837–44. 2002. doi:10.1038/sj.mp.4001093. PMID 12232776.
- ↑ 103.0 103.1 103.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid23527166 - ↑ 104.0 104.1 104.2 104.3 104.4 "Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters". The Journal of Pharmacology and Experimental Therapeutics 359 (1): 159–70. October 2016. doi:10.1124/jpet.116.235838. PMID 27469513.
- ↑ 105.0 105.1 "In vitro biochemical evidence that the psychotomimetics phencyclidine, ketamine and dizocilpine (MK-801) are inactive at cloned human and rat dopamine D2 receptors". European Journal of Pharmacology 540 (1–3): 53–6. July 2006. doi:10.1016/j.ejphar.2006.04.026. PMID 16730695.
- ↑ "Interaction of intravenous anesthetics with recombinant human M1-M3 muscarinic receptors expressed in chinese hamster ovary cells". Anesth Analg 95 (6): 1607–10, table of contents. December 2002. doi:10.1097/00000539-200212000-00025. PMID 12456425.
- ↑ 107.0 107.1 107.2 107.3 107.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid10754635 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9523822 - ↑ "Ketamine potentiates TRPV1 receptor signaling in the peripheral nociceptive pathways". Biochemical Pharmacology 182: 114210. December 2020. doi:10.1016/j.bcp.2020.114210. PMID 32882205.
- ↑ 110.0 110.1 110.2 110.3 "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience 8 (6): 1122–1134. June 2017. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- ↑ "Ketamine: its mechanism(s) of action and unusual clinical uses". British Journal of Anaesthesia 77 (4): 441–4. October 1996. doi:10.1093/bja/77.4.441. PMID 8942324.
- ↑ "Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors". Anesthesiology 86 (4): 903–17. April 1997. doi:10.1097/00000542-199704000-00021. PMID 9105235.
- ↑ "Ketamine and phencyclidine: the good, the bad, and the unexpected". British Journal of Pharmacology 172 (17): 4254–76. September 2015. doi:10.1111/bph.13222. PMID 26075331.
- ↑ 114.0 114.1 "Administration of ketamine for unipolar and bipolar depression". International Journal of Psychiatry in Clinical Practice 21 (1): 2–12. March 2017. doi:10.1080/13651501.2016.1254802. PMID 28097909.
- ↑ 115.0 115.1 "Combination of intravenous S-ketamine and oral tranylcypromine in treatment-resistant depression: A report of two cases". European Neuropsychopharmacology 25 (11): 2183–4. November 2015. doi:10.1016/j.euroneuro.2015.07.021. PMID 26302763.
- ↑ "Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia". Synapse 62 (11): 819–28. November 2008. doi:10.1002/syn.20561. PMID 18720422.
- ↑ "Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil". Synapse 63 (8): 698–704. August 2009. doi:10.1002/syn.20647. PMID 19391150.
- ↑ The Role of Brain Dopamine. Springer Science & Business Media. 6 December 2012. pp. 23–. ISBN 978-3-642-73897-5. https://books.google.com/books?id=yjHwCAAAQBAJ&pg=PA23.
- ↑ "Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses". Archives of General Psychiatry 51 (3): 199–214. March 1994. doi:10.1001/archpsyc.1994.03950030035004. PMID 8122957.
- ↑ "Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men. a randomized, double-blind, placebo-controlled trial". Neuropsychopharmacology 24 (5): 590–3. May 2001. doi:10.1016/S0893-133X(00)00194-9. PMID 11282259.
- ↑ 121.0 121.1 "Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen?". Journal of Psychopharmacology 21 (3): 253–8. May 2007. doi:10.1177/0269881107077767. PMID 17591653.
- ↑ 122.0 122.1 "Ketamine infusions: pharmacokinetics and clinical effects". Br J Anaesth 51 (12): 1167–73. December 1979. doi:10.1093/bja/51.12.1167. PMID 526385.
- ↑ "Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay". Anesth Analg 61 (2): 87–92. February 1982. doi:10.1213/00000539-198202000-00004. PMID 7198883.
- ↑ "Comparative pharmacology of the ketamine isomers. Studies in volunteers". Br J Anaesth 57 (2): 197–203. February 1985. doi:10.1093/bja/57.2.197. PMID 3970799.
- ↑ "Does ketamine metabolite II exist in vivo?". Br J Anaesth 53 (7): 778. July 1981. doi:10.1093/bja/53.7.778. PMID 7248132.
- ↑ Opioid-Induced Hyperalgesia. CRC Press. 19 April 2016. pp. 127–. ISBN 978-1-4200-8900-4. https://books.google.com/books?id=_VrvBQAAQBAJ&pg=PA127.
- ↑ "CYP2B6*6 allele and age substantially reduce steady-state ketamine clearance in chronic pain patients: impact on adverse effects". Br J Clin Pharmacol 80 (2): 276–84. August 2015. doi:10.1111/bcp.12614. PMID 25702819.
- ↑ Synthesis of ketamine references:
- "Amino ketone rearrangements. IV. Thermal rearrangements of α-amino methyl ketones". Journal of Organic Chemistry 30 (9): 2967–72. 1965. doi:10.1021/jo01020a019.
- "Aminoketones and methods for their production" US patent 3254124, issued 31 May 1966, assigned to Parke Davis & Co.
- "Procédé de production d'aminocétones" BE patent 634208, issued 1963-07-15.
- ↑ "[Current aspects of using ketamine in childhood]" (in DE). Anaesthesiologie und Reanimation 23 (3): 64–71. 1998. PMID 9707751.
- ↑ "Mechanistic study on the opposite migration order of the enantiomers of ketamine with α- and β-cyclodextrin in capillary electrophoresis". Journal of Separation Science 25 (15–17): 1155–1166. 2002. doi:10.1002/1615-9314(20021101)25:15/17<1155::AID-JSSC1155>3.0.CO;2-M.
- ↑ Feng N, Vollenweider FX, Minder EI, Rentsch K, Grampp T, Vonderschmitt DJ. Development of a gas chromatography-mass spectrometry method for determination of ketamine in plasma and its application to human samples. Ther. Drug Monit. 17: 95–100, 1995.
- ↑ Parkin MC, Turfus SC, Smith NW, Halket JM, Braithwaite RA, Elliott SP, Osselton MD, Cowan DA, Kicman AT. Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J. Chrom. B 876: 137–142, 2008.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 806–808.
- ↑ "Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581". Anesthesia and Analgesia 45 (1): 29–40. January–February 1966. doi:10.1213/00000539-196601000-00007. PMID 5325977.
- ↑ "Ketamine: 50 Years of Modulating the Mind". Frontiers in Human Neuroscience 10: 612. 2016. doi:10.3389/fnhum.2016.00612. PMID 27965560.
- ↑ 136.0 136.1 "Ketamine". Center for Substance Abuse Research (CESAR); University of Maryland, College Park. 29 October 2013. http://www.cesar.umd.edu/cesar/drugs/ketamine.asp.
- ↑ "Antidepressant effects of ketamine in depressed patients". Biol Psychiatry 47 (4): 351–4. February 2000. doi:10.1016/s0006-3223(99)00230-9. PMID 10686270.
- ↑ "Yale Researchers Study Potential Treatment for Depression in Patients With Parkinson's Disease". NBC Connecticut. 16 March 2022. https://www.nbcconnecticut.com/news/local/yale-researchers-study-potential-treatment-for-depression-in-patients-with-parkinsons-disease/2742209/.
- ↑ "Investigational drugs for treating major depressive disorder". Expert Opinion on Investigational Drugs 26 (1): 9–24. January 2017. doi:10.1080/13543784.2017.1267727. PMID 27960559.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 584–585. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA584.
- ↑ Poisons Standard October 2015 "Poisons Standard". Australian Government. October 2015. https://www.comlaw.gov.au/Details/F2015L01534.
- ↑ Legal status of ketamine in Canada references:
- "Statutes of Canada (S.C.) Controlled Drugs and Substances Act (S.C. 1996 c.19) Schedule I § 14". Government of Canada. 12 June 2014. http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-24.html#h-28.
- "Order Amending Schedule I to the Controlled Drugs and Substances Act". Canada Gazette Part II 139 (19): p. 2130. 21 September 2005. http://napra.ca/Content_Files/Files/CDSA-Ketamine.pdf.
- "Status of ketamine under CDSA". Canadian Society of Customs Brokers. 2 May 2005. http://cscb.ca/node/94386.
- ↑ "Ketamine drug brought under 'Schedule X' to curb abuse". The Times of India. 7 January 2014. http://timesofindia.indiatimes.com/city/goa/Ketamine-drug-brought-under-Schedule-X-to-curb-abuse/articleshow/28486002.cms.
- ↑ "Govt makes notorious 'date rape' drug ketamine harder to buy or sell". The Times of India. 30 December 2013. http://timesofindia.indiatimes.com/india/Govt-makes-notorious-date-rape-drug-ketamine-harder-to-buy-or-sell/articleshow/28116453.cms.
- ↑ Response to ACMD recommendation on ketamine, Crown copyright; Open Government Licence, 12 February 2014, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/279186/ResponseACMDketamineReclassification.pdf, retrieved 21 February 2014.
- ↑ "Party drug ketamine to be upgraded to Class B". The Daily Telegraph. 12 February 2014. https://www.telegraph.co.uk/news/uknews/law-and-order/10633800/Party-drug-ketamine-to-be-upgraded-to-Class-B.html.
- ↑ "Schedules of Controlled Substances: Placement of Ketamine into Schedule III [21 CFR Part 1308. Final Rule 99-17803"]. Federal Register 64 (133): 37673–5. 13 July 1999. http://www.gpo.gov/fdsys/pkg/FR-1999-07-13/pdf/99-17803.pdf.
- ↑ "Acute ketamine intoxication treated by haloperidol: a preliminary study". American Journal of Therapeutics 7 (6): 389–91. November 2000. doi:10.1097/00045391-200007060-00008. PMID 11304647.
- ↑ Drug Abuse. Los Angeles: Health Information Press. 1999. p. 104. ISBN 978-1-885987-11-2. https://archive.org/details/drugabuse00ajam.
- ↑ References for recreational use in literature:
- The Scientist: A Metaphysical Autobiography. Berkeley, CA: Ronin. 1997. pp. 144–. ISBN 978-0-914171-72-0. https://archive.org/details/scientist00lill/page/144.
- The Little Book of Ketamine. Ronin. 2001. pp. 23, 40–45, 46–51, ibid. ISBN 978-1-57951-121-0.
- Journeys Into the Bright World. Rockport, MA: Para Research. 1978. ISBN 978-0-914918-12-7.
- Sisters of the Extreme: Women Writing on the Drug Experience. Inner Traditions. 2000. pp. 254–258, ibid. ISBN 978-0-89281-757-3.
- The Essential Psychedelic Guide. San Francisco: Panther Press. 1994. ISBN 978-0-9642636-1-1.
- ↑ Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. 2001. pp. 50, 89. ISBN 978-0-9660019-3-8.
- ↑ "The Ketamine Necromance". Apocalypse Culture II. Los Angeles: Feral House. 2000. pp. 288–295. https://tranxcend.tumblr.com/post/29813278762/ketamine. Retrieved 18 May 2020.
- ↑ 153.0 153.1 See Max Daly, 2014, "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties," at Vice (online), 23 July 2014, see "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties". 23 July 2014. https://www.vice.com/en_uk/read/ketamine-slowly-ruins-your-bladder-and-kills-you-863., accessed 7 June 2015.
- ↑ "Drug related deaths involving ketamine in England and Wales". A report of the Mortality team, Life Events and Population Sources Division, Office for National Statistics. Government of the United Kingdom. 2013. http://www.ons.gov.uk/ons/about-ons/business-transparency/freedom-of-information/what-can-i-request/published-ad-hoc-data/health/october-2013/drug-related-deaths-involving-ketamine-by-age-group.xls. and "Deaths Related to Drug Poisoning in England and Wales – Office for National Statistics". https://www.ons.gov.uk/ons/rel/subnational-health3/deaths-related-to-drug-poisoning/2012/stb---deaths-related-to-drug-poisoning-2012.html., accessed 7 June 2015.
- ↑ Emma Bowman (15 December 2023). "Matthew Perry died from the 'acute effects of ketamine,' autopsy finds". https://www.npr.org/2023/12/15/1219759019/matthew-perry-cause-of-death.
- ↑ "Do you know... Ketamine". Toronto: Centre for Addiction and Mental Health. 2003. https://knowledgex.camh.net/amhspecialists/resources_families/Pages/ketamine_dyk.aspx.
- ↑ "Ketamine for treatment-resistant depression: When and where is it safe?" (in en). 9 August 2022. https://www.health.harvard.edu/blog/ketamine-for-treatment-resistant-depression-when-and-where-is-it-safe-202208092797.
- ↑ "New Hope for Treatment-Resistant Depression: Guessing Right on Ketamine" (in en). 13 August 2019. https://www.nimh.nih.gov/about/director/messages/2019/new-hope-for-treatment-resistant-depression-guessing-right-on-ketamine.
- ↑ "Ketamine for Treatment-Resistant Depression: a New Advocate". Revista de Investigacion Clinica 70 (2): 65–67. 2018. doi:10.24875/RIC.18002501. PMID 29718013.
- ↑ "mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists". Science 329 (5994): 959–964. August 2010. doi:10.1126/science.1190287. PMID 20724638. Bibcode: 2010Sci...329..959L.
- ↑ Ferreira, Sebastiao Rodrigo; Machado, Alan Rodrigues T.; Furtado, Luís Fernando; Gomes, Jose Hugo de S.; de Almeida, Raquel M.; de Oliveira Mendes, Thiago; Maciel, Valentina N.; Barbosa, Fernando Sergio et al. (2020-10-20). "Ketamine can be produced by Pochonia chlamydosporia: an old molecule and a new anthelmintic?". Parasites & Vectors 13 (1): 527. doi:10.1186/s13071-020-04402-w. ISSN 1756-3305. PMID 33081837.
- ↑ "Pain management in cats—past, present and future. Part 2. Treatment of pain—clinical pharmacology". Journal of Feline Medicine and Surgery 6 (5): 321–33. October 2004. doi:10.1016/j.jfms.2003.10.002. PMID 15363764.
- ↑ "Adjunctive analgesic therapy in veterinary medicine". The Veterinary Clinics of North America. Small Animal Practice 38 (6): 1187–203, v. November 2008. doi:10.1016/j.cvsm.2008.06.002. PMID 18954680.
- ↑ "An outline guide to general anesthesia in exotic species". Veterinary Medicine, Small Animal Clinician 69 (9): 1181–6. September 1974. PMID 4604091.
- ↑ Veterinary Pharmacology and Therapeutics. John Wiley & Sons. 2009. p. 200. ISBN 978-1-118-68590-7. https://books.google.com/books?id=xAPa4WDzAnQC&pg=PP1. Retrieved 26 December 2021.
- ↑ Standard Operating Procedure No. 1 Anesthesia and Analgesia in Rodents, Washington College, 2012, pp. 1–2, https://www.washcoll.edu/live/files/1227-no-1-anesthesia-sop-revised-1012pdf, retrieved 27 November 2015
- ↑ "Guaifenesin: cardiopulmonary effects and plasma concentrations in horses". American Journal of Veterinary Research 41 (11): 1751–5. November 1980. PMID 7212404.
- ↑ "Excitatory actions of propofol and ketamine in the snail Lymnaea stagnalis". Comparative Biochemistry and Physiology. Toxicology & Pharmacology 127 (3): 297–305. December 2000. doi:10.1016/S0742-8413(00)00155-9. PMID 11246501.
External links
- Ketamine fact sheet — from the United States DEA, via Archive.org