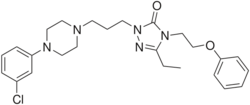
Chemistry:Nefazodone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Serzone, Dutonin, Nefadar, others |
| Other names | BMY-13754-1; MJ-13754-1; MJ-13754; MS-13754 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695005 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20% (variable)[1] |
| Protein binding | 99% (loosely)[1] |
| Metabolism | Liver (CYP3A4, CYP2D6)[2] |
| Metabolites | • Hydroxynefazodone[1] • mCPP[1] • p-Hydroxynefazodone[2] • Triazoledione[1] |
| Elimination half-life | • Nefazodone: 2–4 hours[1] • Hydroxynefazodone: 1.5–4 hours[1] • Triazoledione: 18 hours[1] • mCPP: 4–8 hours[1] |
| Excretion | Urine: 55% Feces: 20–30% |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H32ClN5O2 |
| Molar mass | 470.01 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Nefazodone, sold formerly under the brand names Serzone, Dutonin, and Nefadar among others, is an atypical antidepressant medication which is used in the treatment of depression and for other uses.[3][4][5][6] Nefazodone is still available in the United States,[7] but was withdrawn from other countries due to rare liver toxicity. The medication is taken by mouth.[8]
Side effects of nefazodone include dry mouth, sleepiness, nausea, dizziness, blurred vision, weakness, lightheadedness, confusion, and postural low blood pressure, among others.[8] Rarely, nefazodone can cause serious liver damage, with an incidence of death or liver transplantation of about 1 in every 250,000 to 300,000 patient years.[8] Nefazodone is a phenylpiperazine compound and is related to trazodone. It has been described as a serotonin antagonist and reuptake inhibitor (SARI) due to its combined actions as a potent antagonist of the serotonin 5-HT2A and 5-HT2C receptors and weak serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI).
Nefazodone was introduced for medical use in 1994.[6][9][10] Generic versions were introduced in 2003.[11] Serious liver toxicity was first reported with nefazodone in 1998, and it was withdrawn from most markets by 2004.[10][12] However, as of 2023, it continues to be available in the United States in generic from one manufacturer, Teva Pharmaceuticals[13] and is manufactured in Israel.[14]
Medical uses
Nefazodone is used to treat major depressive disorder, aggressive behavior, anxiety,[15] and panic disorder.[16]
Available forms
Nefazodone is available as 50 mg, 100 mg, 150 mg, 200 mg, and 250 mg tablets for oral ingestion.[17]
Contraindications
Contraindications include the coadministration of terfenadine, astemizole, cisapride, pimozide, or carbamazepine. Nefazodone is contraindicated in patients who were withdrawn from nefazodone because of evident liver injury as well as those that have shown hypersensitivity to the drug, its inactive ingredients, or other phenylpiperazine antidepressants. Furthermore, the coadministration of triazolam and nefazodone should be avoided for all patients, including the elderly, since it causes a significant increase in the plasma level of triazolam and not all commercially available dosage forms of triazolam permit a sufficient dosage reduction. If coadministrated, a 75% reduction in the initial dosage of triazolam is recommended.[17]
Side effects
Common and mild side effects of nefazodone reported in clinical trials more often than placebo include dry mouth (25%), sleepiness (25%), nausea (22%), dizziness (17%), blurred vision (16%), weakness (11%), lightheadedness (10%), confusion (7%), and orthostatic hypotension (5%). Rare and serious adverse reactions may include allergic reactions, fainting, painful/prolonged erection, and jaundice.[8] Nefazodone is not especially associated with increased appetite and weight gain.[18] It is also known for having low levels of sexual side effects in comparisons to SSRIs.[19][20]
Nefazodone can cause severe liver damage which may lead to the need for liver transplantation or to death. The incidence of severe liver damage is approximately 1 in every 250,000 to 300,000 patient-years.[6][8] By the time it started to be withdrawn from the markets in 2003, nefazodone had been associated with at least 53 cases of liver injury (of which 11 led to death) in the United States ,[21] and 51 cases of liver toxicity (of which 2 led to transplantation) in Canada .[22][23] In a 2002 Canadian study of 32 cases, it was noted that databases like those used in the study tended to include only a small proportion of suspected drug reactions.[23]
Treatment protocols suggest screening for pre-existing liver disease before initiating nefazodone, and those with known liver disease should not be prescribed nefazodone. If serum AST or serum ALT levels are more than 3 times the upper limit of normal (ULN), treatment should be permanently withdrawn. Enzyme labs should be done every six months, and nefazodone should not be a first-line treatment.[24]
Interactions
Nefazodone is a potent inhibitor of CYP3A4, and may interact adversely with many commonly used medications that are metabolized by CYP3A4.[25][26][27]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| NET | 360–618 | Human | [28][29] |
| DAT | 360 | Human | [28] |
| 5-HT1A | 80 | Human | [30] |
| 5-HT2A | 26 | Human | [30] |
| 5-HT2C | 72 | Human | [31] |
| α1 | 5.5–48 | Human | [30][29] |
| α1A | 48 | Human | [31] |
| α2 | 84–640 | Human | [30][29] |
| β | >10,000 | Rat | [32] |
| D2 | 910 | Human | [30] |
| H1 | ≥370 | Human | [30][31] |
| mACh | >10,000 | Human | [30] |
| Notes: Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
Nefazodone acts primarily as a potent antagonist of the serotonin 5-HT2A receptor and to a lesser extent of the serotonin 5-HT2C receptor.[30] It also has high affinity for the α1-adrenergic receptor and serotonin 5-HT1A receptor, and relatively lower affinity for the α2-adrenergic receptor and dopamine D2 receptor.[30] Nefazodone has low but significant affinity for the serotonin, norepinephrine, and dopamine transporters as well, and therefore acts as a weak serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI).[28] It has low but potentially significant affinity for the histamine H1 receptor, where it is an antagonist, and hence may have some antihistamine activity.[30][31] Nefazodone has negligible activity at muscarinic acetylcholine receptors, and accordingly, has no anticholinergic effects.[28]
Pharmacokinetics
The bioavailability of nefazodone is low and variable, about 20%.[1] Its plasma protein binding is approximately 99%, but it is bound loosely.[1]
Nefazodone is metabolized in the liver, with the main enzyme involved thought to be CYP3A4.[2] The drug has at least four active metabolites, which include hydroxynefazodone, para-hydroxynefazodone, triazoledione, and meta-chlorophenylpiperazine (mCPP).[1] Nefazodone has a short elimination half-life of about 2 to 4 hours.[1] Its metabolite hydroxynefazodone similarly has an elimination half-life of about 1.5 to 4 hours, whereas the elimination half-lives of triazoledione and mCPP are longer at around 18 hours and 4 to 8 hours, respectively.[1] Due to its long elimination half-life, triazoledione is the major metabolite and predominates in the circulation during nefazodone treatment, with plasma levels that are 4 to 10 times higher than those of nefazodone itself.[1][33] Conversely, hydroxynefazodone levels are about 40% of those of nefazodone at steady state.[1] Plasma levels of mCPP are very low at about 7% of those of nefazodone; hence, mCPP is only a minor metabolite.[1][33] mCPP is thought to be formed from nefazodone specifically by CYP2D6.[2][33]
The ratios of brain-to-plasma concentrations of mCPP to nefazodone are 47:1 in mice and 10:1 in rats, suggesting that brain exposure to mCPP may be much higher than plasma exposure.[1] Conversely, hydroxynefazodone levels in the brain are 10% of those in plasma in rats.[1] As such, in spite of its relatively low plasma concentrations, brain exposure to mCPP may be substantial, whereas that of hydroxynefazodone may be minimal.[1]
Chemistry
Nefazodone is a phenylpiperazine;[34] it is an alpha-phenoxyl derivative of etoperidone which in turn was a derivative of trazodone.[35]
History
Nefazodone was discovered by scientists at Bristol-Myers Squibb (BMS) who were seeking to improve on trazodone by reducing its sedating qualities.[35]
BMS obtained marketing approvals for nefazodone worldwide, including in the United States and Europe, in 1994.[6][9][10] It was marketed in the United States under the brand name Serzone[36] and in Europe under the brand name Dutonin.[37]
The first reports of serious liver toxicity with nefazodone were published in 1998 and 1999.[38][39] These instances were quickly followed by many additional cases.[40][21][22][23]
In 2002 the United States Food and Drug Administration (FDA) obligated BMS to add a black box warning about potential fatal liver toxicity to the drug label.[41][12] Worldwide sales in 2002 were $409 million.[37]
In 2003 Public Citizen filed a citizen petition asking the FDA to withdraw the marketing authorization in the United States, and in early 2004 the organization sued the FDA to attempt to force withdrawal of the drug.[41][42] The FDA issued a response to the petition in June 2004 and filed a motion to dismiss, and Public Citizen withdrew the suit.[42]
Sales of nefazodone were about $100 million in 2003.[43] By that time, it was also being marketed under the additional brand names Serzonil, Nefadar, and Rulivan.[6]
Generic versions were introduced in the United States in 2003[11] and Health Canada withdrew the marketing authorization that same year.[44]
In April 2004, BMS announced that it was going to discontinue the sale of Serzone in the United States in June 2004 and said that this was due to declining sales and generic versions were available in the United States.[11][12][43] By that time, BMS had already withdrawn the drug from the market in Europe, Australia, New Zealand, and Canada.[12]
In August 2020, Teva Pharmaceuticals placed nefazodone in shortage due to a shortage of a raw ingredient. On December 20, 2021, nefazodone was again made available in all strengths.[7][45]
Society and culture
Generic names
Nefazodone is the generic name of the drug and its INN and BAN, while néfazodone is its DCF and nefazodone hydrochloride is its USAN and USP.[3][4][46][5]
Brand names
Nefazodone has been marketed under a number of brand names including Dutonin (AT, ES, IE, UK), Menfazona (ES), Nefadar (CH, DE, NO, SE), Nefazodone BMS (AT), Nefazodone Hydrochloride Teva (US), Reseril (IT), Rulivan (ES), and Serzone (AU, CA, US).[4][5]
Research
Nefazodone was under development for the treatment of panic disorder, and reached phase 3 clinical trials for this indication, but development was discontinued in 2004.[47]
The use of nefazodone to prevent migraine has been studied, due to its antagonism of the serotonin 5-HT2A and 5-HT2C receptors.[48][49][50]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 The American Psychiatric Association Publishing Textbook of Psychopharmacology, Fifth Edition. American Psychiatric Pub. 2017. pp. 460–. ISBN 978-1-58562-523-9. https://books.google.com/books?id=KfHEDgAAQBAJ&pg=PA460.
- ↑ 2.0 2.1 2.2 2.3 Interindividual Variability in Human Drug Metabolism. CRC Press. 24 May 2001. pp. 103–. ISBN 978-0-7484-0864-1. https://books.google.com/books?id=mNKWjla41qUC&pg=PA103.
- ↑ 3.0 3.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 857–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA857.
- ↑ 4.0 4.1 4.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 722–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA722.
- ↑ 5.0 5.1 5.2 "Nefazodone International Brands". Drugs.com. https://www.drugs.com/international/Nefazodone.html.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Drugs of Current Interest: Nefazodone". WHO Pharmaceuticals Newsletter (1). 2003. http://apps.who.int/medicinedocs/en/d/Js4944e/3.html.
- ↑ 7.0 7.1 "Teva Nefazodone Statement" (in en). https://www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Serzone (Nefazodone): Side Effects, Interactions, Warning, Dosage & Uses" (in en). RxList. January 2005. http://www.rxlist.com/serzone-drug.htm.
- ↑ 9.0 9.1 "Nefazodone: a new antidepressant". Am J Health Syst Pharm 52 (24): 2799–812. December 1995. doi:10.1093/ajhp/52.24.2799. PMID 8748566.
- ↑ 10.0 10.1 10.2 "Safety data and withdrawal of hepatotoxic drugs". Therapie 76 (6): 715–723. 2021. doi:10.1016/j.therap.2018.02.004. PMID 29609830.
- ↑ 11.0 11.1 11.2 "Nefazodone" (in en). Drug Patent Watch. https://www.drugpatentwatch.com/p/generic-api/nefazodone+hydrochloride.
- ↑ 12.0 12.1 12.2 12.3 "Anti-Depressant Taken Off Market" (in en). CBS News. April 15, 2004. http://www.cbsnews.com/news/anti-depressant-taken-off-market/.
- ↑ "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process.
- ↑ "DailyMed - NEFAZODONE HYDROCHLORIDE tablet". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=51ff7db5-aaf9-4c3c-86e6-958ebf16b60f.
- ↑ "List of 54 Anxiety Medications Compared" (in en). https://www.drugs.com/condition/anxiety.html.
- ↑ Nefazodone. LiverTox (NIDDK). 2 June 2017. https://livertox.nih.gov/Nefazodone.htm. Retrieved 3 June 2017.
- ↑ 17.0 17.1 "Nefazodone Package insert / prescribing information". Drugs.com. https://www.drugs.com/pro/nefazodone.html.
- ↑ "Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials". The Journal of Clinical Psychiatry 62 (4): 256–260. April 2001. doi:10.4088/JCP.v62n0407. PMID 11379839.
- ↑ "Reemergence of sexual dysfunction in patients with major depressive disorder: double-blind comparison of nefazodone and sertraline". The Journal of Clinical Psychiatry 62 (1): 24–29. January 2001. doi:10.4088/jcp.v62n0106. PMID 11235924.
- ↑ "Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction". The Journal of Clinical Psychiatry 62 (Suppl 3): 10–21. 2001. PMID 11229449. https://pubmed.ncbi.nlm.nih.gov/11229449/. Retrieved 2022-12-17.
- ↑ 21.0 21.1 "Withdrawing drugs: nefazodone, the start of the latest saga". Lancet 361 (9365): 1240. April 2003. doi:10.1016/S0140-6736(03)13030-9. PMID 12699949.
- ↑ 22.0 22.1 "Nefazodone (Serzone) withdrawn because of hepatotoxicity". CMAJ 169 (11): 1187. November 2003. PMID 14638657.
- ↑ 23.0 23.1 23.2 "Hepatic adverse reactions associated with nefazodone". Canadian Journal of Psychiatry 47 (4): 375–377. May 2002. doi:10.1177/070674370204700409. PMID 12025437.
- ↑ "Nefazodone hydrochloride - Drug Summary". PDR.net. https://www.pdr.net/drug-summary/Nefazodone-nefazodone-hydrochloride-661.
- ↑ Lexi-Comp (September 2008). "Nefazodone". The Merck Manual Professional. http://www.merck.com/mmpe/lexicomp/nefazodone.html. Retrieved on November 29, 2008.
- ↑ "Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update". Clinical Therapeutics 30 (7): 1206–1227. July 2008. doi:10.1016/S0149-2918(08)80047-1. PMID 18691982.
- ↑ "Pharmacokinetic drug interactions of new antidepressants: a review of the effects on the metabolism of other drugs". Mayo Clinic Proceedings 72 (9): 835–847. September 1997. doi:10.4065/72.9.835. PMID 9294531.
- ↑ 28.0 28.1 28.2 28.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9537821 - ↑ 29.0 29.1 29.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9400006 - ↑ 30.0 30.1 30.2 30.3 30.4 30.5 30.6 30.7 30.8 30.9 "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology 114 (4): 559–565. May 1994. doi:10.1007/bf02244985. PMID 7855217.
- ↑ 31.0 31.1 31.2 31.3 "Screening the receptorome yields validated molecular targets for drug discovery". Current Pharmaceutical Design 12 (14): 1785–1795. 2006. doi:10.2174/138161206776873680. PMID 16712488.
- ↑ "Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding". Cellular and Molecular Neurobiology 19 (4): 467–489. August 1999. doi:10.1023/A:1006986824213. PMID 10379421.
- ↑ 33.0 33.1 33.2 Antidepressants: Past, Present and Future. Springer Science & Business Media. 6 December 2012. pp. 68–. ISBN 978-3-642-18500-7. https://books.google.com/books?id=Ue3uCAAAQBAJ&pg=PA68.
- ↑ "Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression". Drugs 53 (4): 608–636. April 1997. doi:10.2165/00003495-199753040-00006. PMID 9098663.
- ↑ 35.0 35.1 "Atypical Psychotropic Agents" (in en). Drug Discovery and Development. Springer Science & Business Media. 1987. p. 390. ISBN 9781461248286. https://books.google.com/books?id=O0LuBwAAQBAJ&pg=PA390.
- ↑ Associated Press (16 March 2004). "Consumer group seeks ban on antidepressant" (in en). NBC News. http://www.nbcnews.com/id/4536220/ns/health-mental_health/t/consumer-group-seeks-ban-antidepressant/.
- ↑ 37.0 37.1 "Bristol-Myers to withdraw Dutonin in Europe" (in en). First Word Pharma. January 8, 2003. http://www.firstwordpharma.com/node/213988#axzz4iz3Sls6H.
- ↑ "Nefazodone-induced liver failure: report of three cases". Ann Intern Med 130 (4 Pt 1): 285–8. February 1999. doi:10.7326/0003-4819-130-4-199902160-00013. PMID 10068386.
- ↑ "Adverse effect of nefazodone: hepatitis". Med J Aust 170 (9): 452. May 1999. doi:10.5694/j.1326-5377.1999.tb127827.x. PMID 10341782.
- ↑ "Antidepressant-induced liver injury". Ann Pharmacother 41 (7): 1201–11. July 2007. doi:10.1345/aph.1K114. PMID 17609231.
- ↑ 41.0 41.1 "Public Citizen to sue FDA over Serzone - Pharmaceutical industry news" (in en). The Pharma Letter. 22 March 2004. https://www.thepharmaletter.com/article/public-citizen-to-sue-fda-over-serzone.
- ↑ 42.0 42.1 "Court Decisions and Updates". FDA. https://www.fda.gov/downloads/ICECI/EnforcementActions/EnforcementStory/EnforcementStoryArchive/UCM091483.pdf.
- ↑ 43.0 43.1 "Company Pulls Antidepressant Off Market". WebMD. May 4, 2004. http://www.webmd.com/depression/news/20040520/company-pulls-antidepressant-off-market.
- ↑ "Drug withdrawals from the Canadian market for safety reasons, 1963-2004". CMAJ 172 (6): 765–767. March 2005. doi:10.1503/cmaj.045021. PMID 15767610.
- ↑ "FDA Drug Shortages". https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Nefazodone%20Hydrochloride%20Tablets.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 190–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA190. Retrieved 24 November 2017.
- ↑ "Nefazodone - AdisInsight". https://adisinsight.springer.com/drugs/800000656.
- ↑ "Nefazodone for chronic daily headache prophylaxis: an open-label study". Headache 41 (5): 465–474. May 2001. doi:10.1046/j.1526-4610.2001.01084.x. PMID 11380644.
- ↑ "5-HT2 receptor antagonists and migraine therapy". Journal of Neurology 238 (Suppl 1): S45–S52. 1991. doi:10.1007/BF01642906. PMID 2045831.
- ↑ "Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies". Therapie 60 (5): 441–460. 2005. doi:10.2515/therapie:2005065. PMID 16433010.
External links
 |

