Chemistry:Esreboxetine
From HandWiki
Short description: Chemical compound
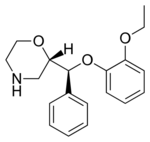 | |
| Clinical data | |
|---|---|
| Other names | AXS-14; PNU-165442G |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C19H23NO3 |
| Molar mass | 313.397 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Esreboxetine (developmental code names AXS-14, PNU-165442G) is a selective norepinephrine reuptake inhibitor which was under development by Pfizer for the treatment of neuropathic pain and fibromyalgia but failed to show significant benefit over currently available medications and was discontinued.[1][2][3][4] It is the (S,S)-(+)-enantiomer of reboxetine and is even more selective as a norepinephrine reuptake inhibitor in comparison.[1][5]
However, recently it has been found that esreboxetine could be effective in fibromyalgia patients.[6]
References
- ↑ 1.0 1.1 Transporters as Targets for Drugs (Topics in Medicinal Chemistry). Berlin: Springer. 2009. ISBN 978-3-540-87911-4. https://books.google.com/books?id=u5nVEyVchwUC&q=esreboxetine&pg=PA81.
- ↑ "Current progress in the pharmacological therapy of fibromyalgia". Expert Opinion on Investigational Drugs 18 (10): 1479–1493. October 2009. doi:10.1517/13543780903203771. PMID 19732029.
- ↑ "Search of esreboxetine". ClinicalTrials.gov. http://clinicaltrials.gov/ct2/results?term=esreboxetine.
- ↑ "Pfizer Stops Work on Esreboxetine for FM". Musculoskeletal Report. New York, NY. 26 February 2009. http://www.mskreport.com/articles.cfm?articleID=3293.
- ↑ "Enantioselective synthesis of (R)- and (S)-N-Boc-morpholine-2-carboxylic acids by enzyme-catalyzed kinetic resolution: application to the synthesis of reboxetine analogs". Tetrahedron Letters 50 (4): 389–391. 2009. doi:10.1016/j.tetlet.2008.11.025.
- ↑ "Safety and efficacy of esreboxetine in patients with fibromyalgia: a fourteen-week, randomized, double-blind, placebo-controlled, multicenter clinical trial". Arthritis and Rheumatism 64 (7): 2387–2397. July 2012. doi:10.1002/art.34390. PMID 22275142.
 |

