Chemistry:Rivanicline
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
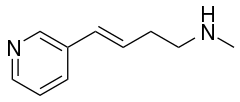
| Formula | C10H14N2 |
| Molar mass | 162.232 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rivanicline (TC-2403, RJR-2403, (E)-metanicotine) is a drug which acts as a partial agonist at neural nicotinic acetylcholine receptors. It is subtype-selective, binding primarily to the α4β2 subtype. It has nootropic effects and was originally developed as a potential treatment for Alzheimer's disease,[1][2][3] but a second action that was subsequently found was that it inhibits the production of Interleukin-8 and thus produces an antiinflammatory effect, and so it has also been developed as a potential treatment for ulcerative colitis.[4] Rivanicline also has stimulant and analgesic actions which are thought to be mediated through stimulation of noradrenaline release,[5] and so it could also have other applications. It has been identified as constituent of tobacco as well.[6][7]
See also
References
- ↑ "Pharmacological Characterization of RJR-2403: A Nicotinic Agonist with Potential Therapeutic Benefit in the Treatment of Alzheimer's Disease". CNS Drug Reviews (Wiley) 3 (4): 325–345. 1997. doi:10.1111/j.1527-3458.1997.tb00331.x. ISSN 1080-563X.
- ↑ "Synthesis of (+/-)-methyl-(1-aryl-4-pyridin-3-yl-but-3-enyl)-amines". Archives of Pharmacal Research 24 (6): 503–7. December 2001. doi:10.1007/bf02975153. PMID 11794523.
- ↑ "Antiamnestic effect of alpha7-nicotinic receptor agonist RJR-2403 in middle-aged ovariectomized rats with Alzheimer type dementia". Bulletin of Experimental Biology and Medicine 142 (6): 700–2. December 2006. doi:10.1007/s10517-006-0455-y. PMID 17603674.
- ↑ "(E)-metanicotine hemigalactarate (TC-2403-12) inhibits IL-8 production in cells of the inflamed mucosa". International Journal of Colorectal Disease 22 (3): 303–12. March 2007. doi:10.1007/s00384-006-0135-4. PMID 16715250.
- ↑ "Nicotinic acetylcholine receptor regulation of spinal norepinephrine release". Anesthesiology 96 (6): 1450–6. June 2002. doi:10.1097/00000542-200206000-00026. PMID 12170059.
- ↑ "The chemical composition of tobacco and tobacco smoke". Chemical Reviews (American Chemical Society (ACS)) 68 (2): 153–207. April 1968. doi:10.1021/cr60252a002. PMID 4868017.
- ↑ Effect of gibberellic acid applications to leaves of Nicotiana on nornicotine, anabasine, metanicotine, oxynicotine, and nicotinic acid content.. https://www.cabdirect.org/cabdirect/abstract/19610300895.
 |

