Chemistry:Dichloropane
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
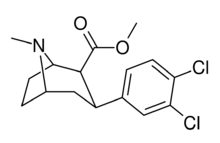
| Formula | C15H17Cl2NO2 |
| Molar mass | 314.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 18, and 0.79 nM, respectively.[1] In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.[2][3]
Methylecgonidine is the direct precursor to this compound.[4]
Trans -CO2Me group
The thermodynamic isomer with a trans -CO2Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials.
- Tesofensine
- Brasofensine
- NS-2359 (GSK-372,475)
See also
- 3,4-DCMP
- O-2390
- List of cocaine analogues
- List of phenyltropanes
References
- ↑ "Synthesis and monoamine transporter binding properties of 3beta-(3',4'-disubstituted phenyl)tropane-2beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry 48 (8): 2767–71. April 2005. doi:10.1021/jm040185a. PMID 15828814.
- ↑ "Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine". Psychopharmacology 153 (1): 103–10. December 2000. doi:10.1007/s002130000602. PMID 11255920.
- ↑ "Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat". Psychopharmacology 159 (1): 58–63. December 2001. doi:10.1007/s002130100891. PMID 11797070.
- ↑ "Synthesis, ligand binding, and QSAR (CoMFA and classical) study of 3 beta-(3'-substituted phenyl)-, 3 beta-(4'-substituted phenyl)-, and 3 beta-(3',4'-disubstituted phenyl)tropane-2 beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry 37 (18): 2865–73. September 1994. doi:10.1021/jm00044a007. PMID 8071935.
 |

