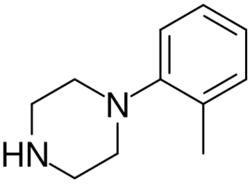
Chemistry:Ortho-Methylphenylpiperazine
 | |
| Clinical data | |
|---|---|
| Other names | oMPP; oMePP; 2-Methylphenylpiperazine; 2-MPP; 2-MePP; 1-(o-Tolyl)piperazine; PAL-169 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H16N2 |
| Molar mass | 176.263 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
ortho-Methylphenylpiperazine (also known as oMPP, oMePP, 1-(2-methylphenyl)piperazine, 2-MPP, and 2-MePP) is a psychoactive designer drug of the phenylpiperazine group.[1][2] It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA), with EC50 values for induction of monoamine release of 175 nM for serotonin, 39.1 nM for norepinephrine, and 296–542 nM for dopamine.[3][4] As such, it has about 4.5-fold preference for induction of norepinephrine release over serotonin, and about 7.6- to 13.9-fold preference for induction of norepinephrine release over dopamine.[3][4]
The 2,3-methyl and 4-methyl analogues show diminished activity as dopamine releasing agents with respective EC50 values of 1,207 nM and 9,523 nM.[3][4] However, at the same time, induction of serotonin and norepinephrine release is retained and more balanced in the 2,3-methyl analogue, with respective EC50 values of 26 nM and 56 nM.[4]
See also
References
- ↑ "Piperazine-like compounds: a new group of designer drugs-of-abuse on the European market". Forensic Sci. Int. 121 (1–2): 47–56. 2001. doi:10.1016/s0379-0738(01)00452-2. PMID 11516887.
- ↑ "Update on 1-benzylpiperazine (BZP) party pills". Arch. Toxicol. 87 (6): 929–47. 2013. doi:10.1007/s00204-013-1057-x. PMID 23685794.
- ↑ 3.0 3.1 3.2 "Cocaine-like discriminative stimulus effects of "norepinephrine-preferring" monoamine releasers: time course and interaction studies in rhesus monkeys". Psychopharmacology 234 (23–24): 3455–3465. 2017. doi:10.1007/s00213-017-4731-5. PMID 28889212.
- ↑ 4.0 4.1 4.2 4.3 "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug Alcohol Depend 147: 1–19. 2015. doi:10.1016/j.drugalcdep.2014.12.005. PMID 25548026.
 |

