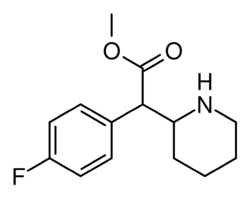
Chemistry:4-Fluoromethylphenidate
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C14H18FNO2 |
| Molar mass | 251.301 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 222.0[1] °C (431.6 °F) |
| |
| |
4-Fluoromethylphenidate (also known as 4-FMPH and 4F-MPH) is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.[2][3][4]
4-Fluoromethylphenidate was studied further along with other analogues of (±)-threo-methylphenidate (TMP) to assess their potential as anti-cocaine medications. 4F-MPH was reported as having an ED50 mg/kg of 0.26 (0.18–0.36), regarding its efficacy as a substitute for cocaine, and a relative potency of 3.33 compared to methylphenidate for the same purpose. This is based on its binding strength to the dopamine transporter vs. its activity as a dopamine reuptake inhibitor. In theory, this would block some of the effects of cocaine, without being as addictive.[5] This has been misinterpreted as a dopamine vs. norepinephrine selectivity ratio.
Another study found that in the threo-isomers of methylphenidate, the meta- and para-substituted compounds with electron-withdrawing substituents tended to have increased binding potency. Compounds containing fluorine, chlorine, bromine and methyl groups were reported to be more potent than methylphenidate as well as the closely related compound 4F-EPH. 4F-MPH was reported as having the following values: [3H]WIN 35428 binding of 35.0 ± 3.0 (2) and [3H]dopamine 142 ± 2.0 (2).[6]
Legal status
4-Fluoromethylphenidate is a Schedule I controlled substance in the US state Alabama.[7] As of 5 May 2017, 4-fluoromethylphenidate is a controlled substance in Canada.[8]
See also
- 3-Bromomethylphenidate
- 3,4-Dichloromethylphenidate
- 4-Fluoroethylphenidate
- 4-Methylmethylphenidate
- Dexmethylphenidate
- HDEP-28
- HDMP-28
- Isopropylphenidate
References
- ↑ "The Drug Enforcement Administration's Special Testing and Research Laboratory Monograph". February 2017. http://www.swgdrug.org/Monographs/4-Fluoromethylphenidate.pdf.
- ↑ "Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites". Bioorganic & Medicinal Chemistry Letters 14 (7): 1799–802. April 2004. doi:10.1016/j.bmcl.2003.12.097. PMID 15026075.
- ↑ "Quantitative structure-activity relationship studies of threo-methylphenidate analogs". Bioorganic & Medicinal Chemistry 18 (20): 7221–38. October 2010. doi:10.1016/j.bmc.2010.08.034. PMID 20846865.
- ↑ "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews 100 (3): 925–1024. March 2000. doi:10.1021/cr9700538. PMID 11749256.
- ↑ "Biochemical and behavioral characterization of novel methylphenidate analogs". The Journal of Pharmacology and Experimental Therapeutics 301 (2): 527–35. May 2002. doi:10.1124/jpet.301.2.527. PMID 11961053.
- ↑ "Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs". Journal of Medicinal Chemistry 39 (6): 1201–9. March 1996. doi:10.1021/jm950697c. PMID 8632426.
- ↑ "Alabama Senate Bill 333 - Controlled substances, Schedule I, additional synthetic controlled substances and analogue substances included in, trafficking in controlled substance analogues, requisite weight increased, Secs. 13A-12-231, 20-2-23 am'd.". March 2014. https://legiscan.com/AL/text/SB333/2014.
- ↑ "Regulations Amending the Food and Drug Regulations (Part G — Methylphenidate)". Health Canada. Government of Canada. April 5, 2017. http://www.gazette.gc.ca/rp-pr/p2/2017/2017-04-05/html/sor-dors43-eng.php.
 |

