Chemistry:Atomoxetine
Atomoxetine, sold under the brand name Strattera, is a medication used to treat attention deficit hyperactivity disorder (ADHD)[10] and, to a lesser extent, cognitive disengagement syndrome.[11][12] It may be used alone or along with psychostimulants.[13][14] It is also used as a cognitive and executive functioning enhancer to improve self-motivation, persistence, attention, inhibition, and working memory.[15][16][17] Use of atomoxetine is only recommended for those who are at least six years old.[10] It is taken orally.[10] Atomoxetine is a selective norepinephrine reuptake inhibitor and is believed to work by increasing norepinephrine and dopamine levels in the brain.[10][8] The effectiveness of atomoxetine is comparable to the commonly prescribed stimulant medication methylphenidate.[18][19][20][21]
Common side effects of atomoxetine include abdominal pain, loss of appetite, nausea, feeling tired, and dizziness.[10] Serious side effects may include angioedema, liver problems, stroke, psychosis, heart problems, suicide, and aggression.[10][22] There is a lack of data regarding its safety during pregnancy; as of 2019, its safety during pregnancy and for use during breastfeeding is not certain.[23][24]
It was approved for medical use in the United States in 2002.[10] In 2020, it was the 287th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[25][26]
Medical uses
Atomoxetine is indicated for the treatment of attention deficit hyperactivity disorder (ADHD).[3]
Attention deficit hyperactivity disorder
Atomoxetine is approved for use in children, adolescents, and adults.[3] However, its efficacy has not been studied in children under six years old.[6] One of the primary differences with the standard stimulant treatments for ADHD is that it has little known abuse potential.[6] Studies and meta-analyses indicate that atomoxetine has comparable efficacy and equal tolerability to methylphenidate in children and adolescents. In adults, efficacy and tolerability are equivalent.[18][19][20][21]
While its efficacy may be less than that of amphetamine,[27] there is some evidence that it may be used in combination with stimulants.[13] Doctors may prescribe non-stimulants including atomoxetine when a person has bothersome side effects from stimulants; when a stimulant was not effective; in combination with a stimulant to increase effectiveness;[28][29] when the cost of stimulants is prohibitive; or when there is concern about the abuse potential of psychostimulants in a patient with a history of drug use disorder.
Unlike α2 adrenoceptor agonists such as guanfacine and clonidine, atomoxetine's use can be abruptly stopped without significant discontinuation effects being seen.[6]
The initial therapeutic effects of atomoxetine usually take 1 to 4 weeks to become apparent.[5][30][31] A further 2 to 4 weeks may be required for the full therapeutic effects to be seen.[32][30] Incrementally increasing response may occur up to 1 year or longer.[31][33] The maximum recommended total daily dose in children and adolescents over 70 kg and adults is 100 mg.[3]
Other uses
Atomoxetine is sometimes used in the treatment of cognitive impairment and frontal lobe symptoms due to conditions like traumatic brain injury (TBI).[34][35] It is used with the goal of treating symptoms like attentional problems, lack of arousal, fatigue, and depression.[34] A 2015 Cochrane review identified only one study of atomoxetine for TBI and found no positive effects.[36] Aside from TBI, atomoxetine was found to be effective in the treatment of akinetic mutism following subarachnoid hemorrhage in a case report.[35][37]
Contraindications
Contraindications include:[6]
- Any cardiovascular disease including:
- Moderate to severe hypertension
- Atrial fibrillation
- Atrial flutter
- Ventricular tachycardia
- Ventricular fibrillation
- Ventricular flutter
- Advanced arteriosclerosis
- Severe cardiovascular disorders
- Uncontrolled hyperthyroidism
- Pheochromocytoma
- Concomitant treatment with monoamine oxidase inhibitors
- Narrow angle glaucoma
Adverse effects
Common side effects include abdominal pain, loss of appetite, nausea, feeling tired, and dizziness.[10] Serious side effects may include angioedema, liver problems, stroke, psychosis, heart problems, suicide, and aggression.[10][22] A 2020 meta-analysis found that atomoxetine was associated with anorexia, weight loss, and hypertension, rating it as a "potentially least preferred agent based on safety" for treating ADHD.[38][39] As of 2019, safety in pregnancy and breastfeeding is not clear;[23] a 2018 review stated that, "[b]ecause of lack of data, the treating physician should consider stopping atomoxetine treatment in women with ADHD during pregnancy."[24]
The U.S. Food and Drug Administration (FDA) has issued a black box warning for suicidal behavior/ideation.[7] Similar warnings have been issued in Australia.[6][40] Unlike stimulant medications, atomoxetine does not have abuse liability or the potential to cause withdrawal effects on abrupt discontinuation.[6][41]
Overdose
Atomoxetine is relatively non-toxic in overdose. Single-drug overdoses involving over 1500 mg of atomoxetine have not resulted in death.[6]
Interactions
Atomoxetine is a substrate for CYP2D6. Concurrent treatment with a CYP2D6 inhibitor such as bupropion, fluoxetine, or paroxetine has been shown to increase plasma atomoxetine by 100% or more, as well as increase N-desmethylatomoxetine levels and decrease plasma 4-hydroxyatomoxetine levels by a similar degree.[42][43][44]
Atomoxetine has been found to directly inhibit hERG potassium currents with an IC50 of 6.3 μM, which has the potential to cause arrhythmia.[43][45] QT prolongation has been reported with atomoxetine at therapeutic doses and in overdose; it is suggested that atomoxetine not be used with other medications that may prolong the QT interval, concomitantly with CYP2D6 inhibitors, and caution to be used in poor metabolizers.[43]
Other notable drug interactions include:[6]
- Antihypertensive agents, due to atomoxetine acting as an indirect sympathomimetic
- Indirect-acting sympathomimetics, such as pseudoephedrine, norepinephrine reuptake inhibitors, or MAOIs
- Direct-acting sympathomimetics, such as phenylephrine or other α1 adrenoceptor agonists, including pressors such as dobutamine or isoprenaline and β2 adrenoceptor agonists
- Highly plasma protein-bound drugs: atomoxetine has the potential to displace these drugs from plasma proteins which may potentiate their adverse or toxic effects. In vitro, atomoxetine does not affect the plasma protein binding of aspirin, desipramine, diazepam, paroxetine, phenytoin, or warfarin[8][46]
Pharmacology
Pharmacodynamics
| Site | ATX | 4-OH-ATX | N-DM-ATX | |
|---|---|---|---|---|
| SERT | 77 | 43 | ND | |
| NET | 5 | 3 | 92 | |
| DAT | 1,451 | ND | ND | |
| 5-HT1A | >1,000 | ND | ND | |
| 5-HT1B | >1,000 | ND | ND | |
| 5-HT1D | >1,000 | ND | ND | |
| 5-HT2 | 2,000 | 1,000 | 1,700 | |
| 5-HT6 | >1,000 | ND | ND | |
| 5-HT7 | >1,000 | ND | ND | |
| α1 | 11,400 | 20,000 | 19,600 | |
| α2A | 29,800 | >30,000 | >10,000 | |
| β1 | 18,000 | 56,100 | 32,100 | |
| M1 | >100,000 | >100,000 | >100,000 | |
| M2 | >100,000 | >100,000 | >100,000 | |
| D1 | >10,000 | >10,000 | >10,000 | |
| D2 | >10,000 | >10,000 | >10,000 | |
| H1 | 12,100 | >100,000 | >100,000 | |
| MOR | Antagonist | 422 | ND | |
| DOR | ND | 300 | ND | |
| KOR | Partial Agonist | 95 | ND | |
| σ1 | >1,000 | ND | ND | |
| GABAA | 200 | >30,000 | >10,000 | |
| NMDA | 0.66 - 3,470a | ND | ND | |
| Kir3.1/3.2 | 10,900b | ND | ND | |
| Kir3.2 | 12,400b | ND | ND | |
| Kir3.1/3.4 | 6,500b | ND | ND | |
| hERG | 6,300 | 20,000 | 5,710 | |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. All values are for human receptors unless otherwise specified. arat cortex. bXenopus oocytes. Additional sources:[47][48][8][46] | ||||
Atomoxetine inhibits the presynaptic norepinephrine transporter (NET), preventing the reuptake of norepinephrine throughout the brain along with inhibiting the reuptake of dopamine in specific brain regions such as the prefrontal cortex, where dopamine transporter (DAT) expression is minimal.[8] In rats, atomoxetine increased prefrontal cortex catecholamine concentrations without altering dopamine levels in the striatum or nucleus accumbens; in contrast, methylphenidate, a dopamine reuptake inhibitor, was found to increase prefrontal, striatal, and accumbal dopamine levels to the same degree.[47] In addition to rats, atomoxetine has also been found to induce similar region-specific catecholamine level alteration in mice.[49]
Atomoxetine's status as a serotonin transporter (SERT) inhibitor at clinical doses in humans is uncertain. A PET imaging study on rhesus monkeys found that atomoxetine occupied >90% and >85% of neural NET and SERT, respectively.[50] However, both mouse and rat microdialysis studies have failed to find an increase in extracellular serotonin in the prefrontal cortex following acute or chronic atomoxetine treatment.[47][49] Supporting atomoxetine's selectivity, a human study found no effects on platelet serotonin uptake (a marker of SERT inhibition) and inhibition of the pressor effects of tyramine (a marker of NET inhibition).[51]
Atomoxetine has been found to act as an NMDA receptor antagonist in rat cortical neurons at therapeutic concentrations.[52][53] It causes a use-dependent open-channel block and its binding site overlaps with the Mg2+ binding site.[52][53] Atomoxetine's ability to increase prefrontal cortex firing rate in anesthetized rats could not be blocked by D1 or α1-adrenergic receptor antagonists, but could be potentiated by NMDA or an α2-adrenergic receptor antagonist, suggesting a glutaminergic mechanism.[54] In Sprague Dawley rats, atomoxetine reduces NR2B protein content without altering transcript levels.[55] Aberrant glutamate and NMDA receptor function have been implicated in the etiology of ADHD.[56][57]
Atomoxetine also reversibly inhibits GIRK currents in Xenopus oocytes in a concentration-dependent, voltage-independent, and time-independent manner.[58] Kir3.1/3.2 ion channels are opened downstream of M2, α2, D2, and A1 stimulation, as well as other Gi-coupled receptors.[58] Therapeutic concentrations of atomoxetine are within range of interacting with GIRKs, especially in CYP2D6 poor metabolizers.[58] It is not known whether this contributes to the therapeutic effects of atomoxetine in ADHD.
4-Hydroxyatomoxetine, the major active metabolite of atomoxetine in CYP2D6 extensive metabolizers, has been found to have sub-micromolar affinity for opioid receptors, acting as an antagonist at μ-opioid receptors and a partial agonist at κ-opioid receptors.[48] It is not known whether this action at the kappa-opioid receptor leads to CNS-related adverse effects.
Pharmacokinetics
Orally administered atomoxetine is rapidly and completely absorbed.[8] First-pass metabolism by the liver is dependent on CYP2D6 activity, resulting in an absolute bioavailability of 63% for extensive metabolizers and 94% for poor metabolizers.[8] Maximum plasma concentration is reached in 1–2 hours.[8] If taken with food, the maximum plasma concentration decreases by 10-40% and delays the tmax by 3 hours.[8] Drugs affecting gastric pH have no effect on oral bioavailability.[3]
Following intravenous delivery, atomoxetine has a volume of distribution of 0.85 L/kg (indicating distribution primarily in total body water), with limited partitioning into red blood cells.[8][59] It is highly bound to plasma proteins (98.7%), mainly albumin, along with α1-acid glycoprotein (77%) and IgG (15%).[8][46] Its metabolite N-desmethylatomoxetine is 99.1% bound to plasma proteins, while 4-hydroxyatomoxetine is only 66.6% bound.[8]
The half-life of atomoxetine varies widely between individuals, with an average range of 4.5 to 19 hours.[8][9] As atomoxetine is metabolized by CYP2D6, exposure may be increased 10-fold in CYP2D6 poor metabolizers.[9] Among CYP2D6 extensive metabolizers, the half-life of atomoxetine averaged 5.34 hours and the half-life of the active metabolite N-desmethylatomoxetine was 8.9 hours.[8][60] By contrast, among CYP2D6 poor metabolizers the half-life of atomoxetine averaged 20.0 hours and the half-life of N-desmethylatomoxetine averaged 33.3 hours.[8][60] Steady-state levels of atomoxetine are typically achieved at or around day 10 of regular dosing, with trough plasma concentrations (Ctrough) residing around 30–40°ng/mL; however, both the time to steady-state levels and Ctrough are expected to vary based on a patient's CYP2D6 profile.[61][62]
Atomoxetine, N-desmethylatomoxetine, and 4-hydroxyatomoxetine produce minimal to no inhibition of CYP1A2 and CYP2C9, but inhibit CYP2D6 in human liver microsomes at concentrations between 3.6 and 17 μmol/L.[citation needed] Plasma concentrations of 4-hydroxyatomoxetine and N-desmethylatomoxetine at steady state are 1.0% and 5% that of atomoxetine in CYP2D6 extensive metabolizers, and are 5% and 45% that of atomoxetine in CYP2D6 poor metabolizers.[3]
Atomoxetine is excreted unchanged in urine at <3% in both extensive and poor CYP2D6 metabolizers, with >96% and 80% of a total dose being excreted in urine, respectively.[8] The fractions excreted in urine as 4-hydroxyatomoxetine and its glucuronide account for 86% of a given dose in extensive metabolizers, but only 40% in poor metabolizers.[8] CYP2D6 poor metabolizers excrete greater amounts of minor metabolites, namely N-desmethylatomoxetine and 2-hydroxymethylatomoxetine and their conjugates.[8]
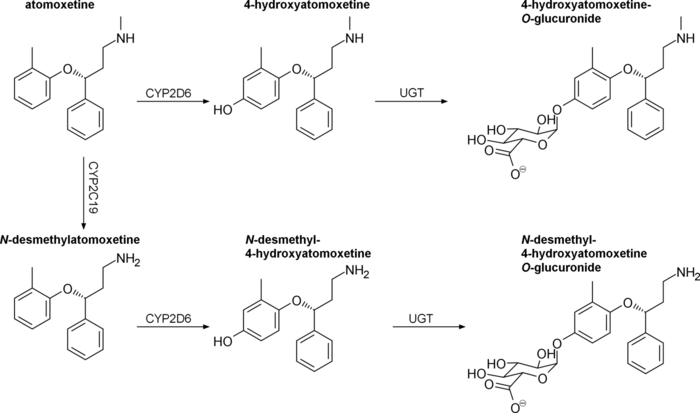
Pharmacogenomics
Chinese adults homozygous for the hypoactive CYP2D6*10 allele have been found to exhibit two-fold higher area-under-the-curve (AUCs) and 1.5-fold higher maximum plasma concentrations compared to extensive metabolizers.[8]
Japanese men homozygous for CYP2D6*10 have similarly been found to experience two-fold higher AUCs compared to extensive metabolizers.[8]
Chemistry
Atomoxetine, or (−)-methyl[(3R)-3-(2-methylphenoxy)-3-phenylpropylamine, is a white, granular powder that is highly soluble in water.
Synthesis
Detection in biological fluids
Atomoxetine may be quantitated in plasma, serum or whole blood in order to distinguish extensive versus poor metabolizers in those receiving the drug therapeutically, to confirm the diagnosis in potential poisoning victims or to assist in the forensic investigation in a case of fatal overdosage.[65]
History
Atomoxetine is manufactured, marketed, and sold in the United States as the hydrochloride salt (atomoxetine HCl) under the brand name Strattera by Eli Lilly and Company, the original patent-filing company and current U.S. patent owner. Atomoxetine was initially intended to be developed as an antidepressant, but it was found to be insufficiently efficacious for treating depression. It was, however, found to be effective for ADHD and was approved by the FDA in 2002, for the treatment of ADHD. Its patent expired in May 2017.[66] On 12 August 2010, Lilly lost a lawsuit that challenged its patent on Strattera, increasing the likelihood of an earlier entry of a generic into the US market.[67] On 1 September 2010, Sun Pharmaceuticals announced it would begin manufacturing a generic in the United States.[68] In a 29 July 2011 conference call, however, Sun Pharmaceutical's Chairman stated "Lilly won that litigation on appeal so I think [generic Strattera]'s deferred."[69]
In 2017 the FDA approved the generic production of atomoxetine by four pharmaceutical companies.[70]
Society and culture
The drug was originally known as tomoxetine. It was renamed to avoid medication errors, as the name may be confused with tamoxifen.[71]
Brand names
In India, atomoxetine is sold under brand names including Axetra, Axepta, Attera, Tomoxetin, and Attentin. In Australia, Canada, Italy, Portugal, Romania, Spain, Switzerland and the US, atomoxetine is sold under the brand name Strattera. In the Czech Republic it is sold under brand names including Mylan. In Poland, it is sold under the brand name Auroxetyn. In Iran, atomoxetine is sold under brand names including Stramox. In 2017, a generic version was approved in the United States.[70]
Research
There has been some suggestion that atomoxetine might be a helpful adjunct in people with major depression, particularly in cases with concomitant ADHD.[72]
Atomoxetine may be used in those with ADHD and bipolar disorder although such use has not been well studied.[73] Some benefit has also been seen in people with ADHD and autism.[74] As with other norepinephrine reuptake inhibitors it appears to reduce anxiety and depression symptoms, although attention has focused mainly on specific patient groups such as those with concurrent ADHD[75] or methamphetamine dependence.[76]
Cognitive disengagement syndrome
Some studies indicate that atomoxetine is effective for treatment of cognitive disengagement syndrome.[11]
References
- ↑ "Atomoxetine (Strattera) Use During Pregnancy". 22 August 2019. https://www.drugs.com/pregnancy/atomoxetine.html.
- ↑ "Strattera 10mg hard capsules - Summary of Product Characteristics (SmPC)". 8 February 2021. https://www.medicines.org.uk/emc/product/5531/smpc.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Strattera- atomoxetine hydrochloride capsule". Eli Lilly and Company. 29 January 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=309de576-c318-404a-bc15-660c2b1876fb.
- ↑ "Active substance(s): atomoxetine". List of nationally authorised medicinal products. European Medicines Agency. 2016. https://www.ema.europa.eu/en/documents/psusa/atomoxetine-list-nationally-authorised-medicinal-products-psusa/00000262/201511_en.pdf.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "atomoxetine (Rx) – Strattera". Medscape Reference. WebMD. http://reference.medscape.com/drug/strattera-atomoxetine-342994.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 "Strattera (atomoxetine hydrochloride)". TGA eBusiness Services. Eli Lilly Australia Pty. Limited. 21 August 2013. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-04269-3.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Atomoxetine Hydrochloride capsule [Mylan Pharmaceuticals Inc."]. DailyMed. Mylan Pharmaceuticals Inc.. October 2011. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bdab7205-d0ff-41c3-8430-2d399dfaa759.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 8.15 8.16 8.17 8.18 8.19 8.20 "Clinical pharmacokinetics of atomoxetine". Clinical Pharmacokinetics 44 (6): 571–590. 2005. doi:10.2165/00003088-200544060-00002. PMID 15910008.
- ↑ 9.0 9.1 9.2 "Atomoxetine pharmacogenetics: associations with pharmacokinetics, treatment response and tolerability". Pharmacogenomics 16 (13): 1513–1520. 2015. doi:10.2217/PGS.15.93. PMID 26314574.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 "Atomoxetine Hydrochloride Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/atomoxetine-hydrochloride.html.
- ↑ 11.0 11.1 "Atomoxetine-Related Change in Sluggish Cognitive Tempo Is Partially Independent of Change in Attention-Deficit/Hyperactivity Disorder Inattentive Symptoms". Journal of Child and Adolescent Psychopharmacology 27 (1): 38–42. February 2017. doi:10.1089/cap.2016.0115. PMID 27845858.
- ↑ "Report of a Work Group on Sluggish Cognitive Tempo: Key Research Directions and a Consensus Change in Terminology to Cognitive Disengagement Syndrome". Journal of the American Academy of Child and Adolescent Psychiatry 62 (6): 629–645. June 2023. doi:10.1016/j.jaac.2022.07.821. PMID 36007816.
- ↑ 13.0 13.1 "A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability". Journal of Child and Adolescent Psychopharmacology 23 (3): 179–193. April 2013. doi:10.1089/cap.2012.0093. PMID 23560600.
- ↑ "Parent's Medication Guide: ADHD". American Psychiatric Association & American Academy of Child and Adolescent Psychiatry (AACAP). June 2013. https://www.psychiatry.org/patients-families/adhd/what-is-adhd. "Though not FDA-approved for combined treatment, atomoxetine (Strattera) is sometimes used in conjunction with stimulants as an off-label combination therapy."
- ↑ "14: Higher Cognitive Function and Behavioral Control". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3 ed.). New York: McGraw-Hill Medical. 2015. ISBN 9780071827706.
- ↑ "Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents: A Systematic Review". JAMA 315 (18): 1997–2008. May 2016. doi:10.1001/jama.2016.5453. PMID 27163988.
- ↑ "Cognitive enhancement as a treatment for drug addictions". Neuropharmacology 64 (1): 452–463. January 2013. doi:10.1016/j.neuropharm.2012.06.021. PMID 22735770.
- ↑ 18.0 18.1 Hanwella, Raveen; Senanayake, Madhri; de Silva, Varuni (2011-11-10). "Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis". BMC Psychiatry 11: 176. doi:10.1186/1471-244X-11-176. ISSN 1471-244X. PMID 22074258.
- ↑ 19.0 19.1 "Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review and meta-analysis". Medical Journal of the Islamic Republic of Iran 30: 325. 10 February 2016. PMID 27390695.
- ↑ 20.0 20.1 Hazell, Philip L.; Kohn, Michael R.; Dickson, Ruth; Walton, Richard J.; Granger, Renee E.; van Wyk, Gregory W. (November 2011). "Core ADHD Symptom Improvement With Atomoxetine Versus Methylphenidate: A Direct Comparison Meta-Analysis" (in en). Journal of Attention Disorders 15 (8): 674–683. doi:10.1177/1087054710379737. ISSN 1087-0547. PMID 20837981. http://journals.sagepub.com/doi/10.1177/1087054710379737. Retrieved 7 December 2023.
- ↑ 21.0 21.1 Bushe, Chris; Day, Kathleen; Reed, Victoria; Karlsdotter, Kristina; Berggren, Lovisa; Pitcher, Ashley; Televantou, Foula; Haynes, Virginia (May 2016). "A network meta-analysis of atomoxetine and osmotic release oral system methylphenidate in the treatment of attention-deficit/hyperactivity disorder in adult patients" (in en). Journal of Psychopharmacology 30 (5): 444–458. doi:10.1177/0269881116636105. ISSN 0269-8811. PMID 27005307. http://journals.sagepub.com/doi/10.1177/0269881116636105. Retrieved 7 December 2023.
- ↑ 22.0 22.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 344–345. ISBN 9780857113382.
- ↑ 23.0 23.1 "Atomoxetine Pregnancy and Breastfeeding Warnings". https://www.drugs.com/pregnancy/atomoxetine.html.
- ↑ 24.0 24.1 "Pharmacological Treatment of Attention Deficit Hyperactivity Disorder During Pregnancy and Lactation". Pharmaceutical Research 35 (3): 46. February 2018. doi:10.1007/s11095-017-2323-z. PMID 29411149.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Atomoxetine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Atomoxetine.
- ↑ Adult ADHD Diagnostic Assessment and Treatment. Springer London. 2013. doi:10.1007/978-1-4471-4138-9. ISBN 978-1-4471-4137-2.
- ↑ "Attention Deficit Hyperactivity Disorder". National Institute of Mental Health (NIMH). https://www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- ↑ "Mental Health Medications". https://www.nimh.nih.gov/health/topics/mental-health-medications/index.shtml#part_149861.
- ↑ 30.0 30.1 "Systematic review of atomoxetine data in childhood and adolescent attention-deficit hyperactivity disorder 2009-2011: focus on clinical efficacy and safety". J Psychopharmacol 28 (3): 204–11. March 2014. doi:10.1177/0269881113478475. PMID 23438503.
- ↑ 31.0 31.1 "A critical appraisal of atomoxetine in the management of ADHD". Ther Clin Risk Manag 12: 27–39. 2016. doi:10.2147/TCRM.S59270. PMID 26730199.
- ↑ The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. 2012. ISBN 978-0-470-97948-8.
- ↑ "Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients". J Psychopharmacol 29 (12): 1221–30. December 2015. doi:10.1177/0269881115602489. PMID 26349559.
- ↑ 34.0 34.1 "Atomoxetine for individuals with traumatic brain injury". J Head Trauma Rehabil 21 (1): 85–8. 2006. doi:10.1097/00001199-200601000-00010. PMID 16456396.
- ↑ 35.0 35.1 "On the pathophysiology and treatment of akinetic mutism". Neurosci Biobehav Rev 112: 270–278. May 2020. doi:10.1016/j.neubiorev.2020.02.006. PMID 32044373.
- ↑ "Pharmacotherapy for chronic cognitive impairment in traumatic brain injury". Cochrane Database Syst Rev (12): CD009221. December 2015. doi:10.1002/14651858.CD009221.pub2. PMID 26624881.
- ↑ "Treatment of chronic akinetic mutism with atomoxetine: subtraction analysis of brain f-18 fluorodeoxyglucose positron emission tomographic images before and after medication: a case report". Clin Neuropharmacol 33 (4): 209–11. July 2010. doi:10.1097/WNF.0b013e3181dca948. PMID 20661027.
- ↑ "Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects". World Psychiatry 19 (2): 214–232. June 2020. doi:10.1002/wps.20765. PMID 32394557.
- ↑ "Psychiatric drugs given to children and adolescents have been ranked in order of safety". NIHR Evidence. 1 September 2020. doi:10.3310/alert_40795. https://evidence.nihr.ac.uk/alert/psychiatric-drugs-given-to-children-and-adolescents-have-been-ranked-in-order-of-safety/. Retrieved 12 March 2022.
- ↑ "Atomoxetine and suicidality in children and adolescents". Australian Prescriber 4 (5): 166. October 2013. http://www.australianprescriber.com/magazine/36/5/166/9. Retrieved 10 November 2013.
- ↑ "Changes in symptoms and adverse events after discontinuation of atomoxetine in children and adults with attention deficit/hyperactivity disorder: a prospective, placebo-controlled assessment". Journal of Clinical Psychopharmacology 24 (1): 30–35. February 2004. doi:10.1097/01.jcp.0000104907.75206.c2. PMID 14709944.
- ↑ "Evaluation of a Potential Metabolism-Mediated Drug-Drug Interaction Between Atomoxetine and Bupropion in Healthy Volunteers". Journal of Pharmacy & Pharmaceutical Sciences 19 (2): 198–207. April–June 2016. doi:10.18433/j3h03r. PMID 27518170.
- ↑ 43.0 43.1 43.2 "Cardiovascular side effects of atomoxetine and its interactions with inhibitors of the cytochrome p450 system". Case Reports in Medicine 2011: 952584. 2011. doi:10.1155/2011/952584. PMID 21765848.
- ↑ "Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics". Journal of Clinical Pharmacology 42 (11): 1219–27. November 2002. doi:10.1177/009127002762491307. PMID 12412820.
- ↑ "Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents". British Journal of Pharmacology 156 (2): 226–36. January 2009. doi:10.1111/j.1476-5381.2008.00018.x. PMID 19154426.
- ↑ 46.0 46.1 46.2 "21-411 Strattera Clinical Pharmacology Biopharmaceutics Review Part 2". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-411_Strattera_biopharmr_P2.pdf.
- ↑ 47.0 47.1 47.2 "Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder". Neuropsychopharmacology 27 (5): 699–711. November 2002. doi:10.1016/S0893-133X(02)00346-9. PMID 12431845.
- ↑ 48.0 48.1 "Synthesis and biological evaluation of the major metabolite of atomoxetine: elucidation of a partial kappa-opioid agonist effect". Bioorganic & Medicinal Chemistry Letters 14 (15): 4083–5. August 2004. doi:10.1016/j.bmcl.2004.05.018. PMID 15225731.
- ↑ 49.0 49.1 "Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice". Journal of Neurochemistry 114 (1): 259–70. July 2010. doi:10.1111/j.1471-4159.2010.06750.x. PMID 20403082.
- ↑ "Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: Implications on treatment of depression and ADHD". NeuroImage 86: 164–71. February 2014. doi:10.1016/j.neuroimage.2013.08.001. PMID 23933039. "The noradrenergic action also exerts an important clinical effect in different antidepressant classes such as desipramine and nortriptyline (tricyclics, prevalent noradrenergic effect), reboxetine and atomoxetine (relatively pure noradrenergic reuptake inhibitor (NRIs)), and dual action antidepressants such as the serotonin noradrenaline reuptake inhibitors (SNRIs), the noradrenergic and dopaminergic reuptake inhibitor (NDRI) bupropion, and other compounds (e.g., mianserin, mirtazapine), which enhance the noradrenergic transmission".
- ↑ "Clinical pharmacology of tomoxetine, a potential antidepressant". The Journal of Pharmacology and Experimental Therapeutics 232 (1): 139–43. January 1985. PMID 3965689.
- ↑ 52.0 52.1 "Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations". British Journal of Pharmacology 160 (2): 283–91. May 2010. doi:10.1111/j.1476-5381.2010.00707.x. PMID 20423340.
- ↑ 53.0 53.1 "Inhibition of the NMDA and AMPA receptor channels by antidepressants and antipsychotics". Brain Research 1660: 58–66. April 2017. doi:10.1016/j.brainres.2017.01.028. PMID 28167075.
- ↑ "Psychostimulants and atomoxetine alter the electrophysiological activity of prefrontal cortex neurons, interaction with catecholamine and glutamate NMDA receptors". Psychopharmacology 232 (12): 2191–205. June 2015. doi:10.1007/s00213-014-3849-y. PMID 25572531.
- ↑ "Atomoxetine affects transcription/translation of the NMDA receptor and the norepinephrine transporter in the rat brain--an in vivo study". Drug Design, Development and Therapy 7: 1433–46. 2013. doi:10.2147/DDDT.S50448. PMID 24348020.
- ↑ "Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study". Translational Psychiatry 4 (3): e373. March 2014. doi:10.1038/tp.2014.11. PMID 24643164.
- ↑ "Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation". Current Pharmaceutical Design 20 (32): 5180–5. 2014. doi:10.2174/1381612819666140110115227. PMID 24410567.
- ↑ 58.0 58.1 58.2 "Inhibition of G-protein-activated inwardly rectifying K+ channels by the selective norepinephrine reuptake inhibitors atomoxetine and reboxetine". Neuropsychopharmacology 35 (7): 1560–9. June 2010. doi:10.1038/npp.2010.27. PMID 20393461.
- ↑ "atomoxetine HC". https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021411s004s012s013s015s021lbl.pdf.
- ↑ 60.0 60.1 "Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism". Drug Metabolism and Disposition 31 (1): 98–107. January 2003. doi:10.1124/dmd.31.1.98. PMID 12485958.
- ↑ "Clinical pharmacokinetics of atomoxetine". Clinical Pharmacokinetics 44 (6): 571–590. 2005. doi:10.2165/00003088-200544060-00002. PMID 15910008.
- ↑ "Atomoxetine" (in en). Encyclopedia of Psychopharmacology. Berlin, Heidelberg: Springer. 2010. pp. 158–160. doi:10.1007/978-3-540-68706-1_35. ISBN 978-3-540-68706-1.
- ↑ & Klaus K. Schmiegel"Aryloxyphenylpropylamines in treating depression" A US patent 4018895 A, published 19 April 1977, assigned to Eli Lilly And Company
- ↑ & Edward Ralph Lavagnino"3-aryloxy-3-phenylpropylamines" B1 US patent EP0052492 B1, published 29 February 1984, assigned to Eli Lilly And Company
- ↑ Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. 2008. pp. 118–20. ISBN 978-0-931890-08-6. https://archive.org/details/dispositionoftoxe2base/page/118.
- ↑ "Patent and Exclusivity Search Results". Electronic Orange Book. U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021411&Product_No=002&table1=OB_Rx.
- ↑ "Drugmaker Eli Lilly loses patent case over ADHD drug, lowers revenue outlook". Chicago Tribune. http://www.chicagotribune.com/business/sns-ap-us-eli-lilly-patent,0,7016147.story.[yes|permanent dead link|dead link}}]
- ↑ "Sun Pharma receives USFDA approval for generic Strattera capsules". International Business Times. http://www.ibtimes.com/articles/48022/20100901/sun-pharma-usfda-strattera-capsules-eli-lilly-atomoxetine-hydrochloride-attention-deficit-hyperactiv.htm.
- ↑ "Sun Pharma Q1 2011-12 Earnings Call Transcript 10.00 am, July 29, 2011". http://www.sunpharma.com/images/finance/FY12%20Q1%20Earnings%20Call%20Transcript.pdf.
- ↑ 70.0 70.1 "FDA approves first generic Strattera for the treatment of ADHD". U.S. Food and Drug Administration (FDA) (Press release). 30 May 2017. Archived from the original on 4 June 2017. Retrieved 1 January 2018.
- ↑ "Atomoxetine: a novel treatment for child and adult ADHD". Neuropsychiatric Disease and Treatment 2 (4): 455–466. December 2006. doi:10.2147/nedt.2006.2.4.455. PMID 19412494.
- ↑ Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. 2015. ISBN 9780071827706.[page needed]
- ↑ "The use of stimulants and atomoxetine in adults with comorbid ADHD and bipolar disorder". Expert Opinion on Pharmacotherapy 16 (14): 2193–204. 2015. doi:10.1517/14656566.2015.1079620. PMID 26364896.
- ↑ Autism Spectrum Disorder Parents Medication Guide. Washington, DC: American Academy of Child and Adolescent Psychiatry. 2016. pp. 13. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/autism/Autism_Spectrum_Disorder_Parents_Medication_Guide.pdf. "Atomoxetine (Strattera) has also been researched in controlled studies for treatment of ADHD in children with autism, and showed some improvements, particularly for hyperactivity and impulsivity"
- ↑ "Anxiety reduction on atomoxetine and methylphenidate medication in children with ADHD". Pediatrics International 58 (6): 476–81. June 2016. doi:10.1111/ped.12847. PMID 26579704.
- ↑ "Atomoxetine Efficacy in Methamphetamine Dependence during Methadone Maintenance Therapy". Archives of Iranian Medicine 22 (12): 692–698. December 2019. PMID 31823620.
Further reading
- "Atomoxetine Therapy and CYP2D6 Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2015. Bookshelf ID: NBK315951. https://www.ncbi.nlm.nih.gov/books/NBK315951/. Retrieved 7 February 2020.
 |