Chemistry:Devapamil
| |
| Names | |
|---|---|
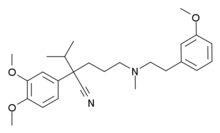
| IUPAC name
(RS)-2-(3,4-dimethoxyphenyl)-2-isopropyl-5-[2-(3-methoxyphenyl)ethyl-methylamino]pentanenitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H36N2O3 | |
| Molar mass | 424.57564 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Devapamil is a calcium channel blocker. It is also known as desmethoxyverapamil, which is a phenylalkylamine (PAA) derivative.[1] Devapamil not only inhibits by blocking the calcium gated channels, but also by depolarizing the membrane during the sodium-potassium exchanges.[2]
Structure
Devapamil consists of two aromatic rings with methoxy substituents connected by an alkylamine chain increasing flexibility and overall potency.[3]
Animal studies
Devapamil in rats can be used to decrease glutathione levels and increase oxidation of lipids, which makes it effective in preclusion of ulcers caused by stress.[4][5] The medical characteristics of this drug, and other phenylalkylamines, depends greatly on the state of the calcium channels being targeted which results in a greater affinity and drug efficiency. [6]
References
- ↑ "The effect of the phenylalkylamine D888 (devapamil) on force and Ca2+ current in isolated frog skeletal muscle fibres". The Journal of Physiology 413 (1): 521–41. June 1989. doi:10.1113/jphysiol.1989.sp017667. PMID 2557440.
- ↑ "L-type Ca2+ channel antagonists block voltage-dependent Ca2+ channels in identified leech neurons". Brain Research 1013 (2): 159–67. July 2004. doi:10.1016/j.brainres.2004.03.038. PMID 15193524.
- ↑ "Structural model for phenylalkylamine binding to L-type calcium channels". The Journal of Biological Chemistry 284 (41): 28332–42. October 2009. doi:10.1074/jbc.M109.027326. PMID 19700404.
- ↑ "Gastric lipid peroxidation, glutathione and calcium channel blockers in the stress-induced ulcer model in rats". Pharmacological Research 30 (2): 123–35. August 1994. doi:10.1016/1043-6618(94)80004-9. PMID 7816741.
- ↑ "Protective effects of lysozyme chloride and reduced glutathione on betel quid chewing-produced gastric oxidative stress and haemorrhagic ulcer in rats". Inflammopharmacology 12 (2): 115–29. May 2004. doi:10.1163/1568560041352284. PMID 15265315.
- ↑ "Structural model for phenylalkylamine binding to L-type calcium channels". The Journal of Biological Chemistry 284 (41): 28332–42. October 2009. doi:10.1074/jbc.M109.027326. PMID 19700404.
 |

