Chemistry:Agomelatine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Melitor, Thymanax, Valdoxan, others |
| Other names | AGO-178; AGO178C; S-20098; S-20098-F55 |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Dependence liability | Low[1] |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 1%[2] |
| Protein binding | 95%[2] |
| Metabolism | Liver (90% CYP1A2 and 10% CYP2C9)[2] |
| Elimination half-life | 1–2 hours[2] |
| Excretion | Kidney (80%, mostly as metabolites)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H17NO2 |
| Molar mass | 243.306 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Agomelatine, sold under the brand names Valdoxan and Thymanax, among others, is an atypical antidepressant most commonly used to treat major depressive disorder and generalized anxiety disorder.[6] One review found that it is as effective as other antidepressants with similar discontinuation rates overall but fewer discontinuations due to side effects.[6][7] Another review also found it was similarly effective to many other antidepressants.[8]
Common side effects include weight gain, fatigue, liver problems, nausea, headaches, and anxiety.[9][10] Due to potential liver problems ongoing blood tests are recommended.[11] Its use is not recommended in people with dementia or over the age of 75.[9] There is tentative evidence that it may have fewer side effects than some other antidepressants.[6] It acts by blocking certain serotonin receptors and activating melatonin receptors.[11]
Agomelatine was approved for medical use in Europe in 2009 and Australia in 2010.[11] Its use is not approved in the United States and efforts to get approval were ended in 2011.[11] It was developed by the pharmaceutical company Servier.[11]
Medical uses
Major depressive disorder
Agomelatine is used for the treatment of major depressive episodes in adults in Europe.[12] Ten placebo controlled trials have been performed to investigate the short term efficacy of agomelatine in major depressive disorder. At the end of treatment, significant efficacy was demonstrated in six of the ten short-term double-blind placebo-controlled studies.[12] Two were considered "failed" trials, as comparators of established efficacy failed to differentiate from placebo. Efficacy was also observed in more severely depressed patients in all positive placebo-controlled studies.[12] The maintenance of antidepressant efficacy was demonstrated in a relapse prevention study.[12] One meta-analysis found agomelatine to be as effective as standard antidepressants, with an effect size (SMD) of 0.24.[7][13]
In 2018, a systematic review and network meta-analysis comparing the efficacy and acceptability of 21 antidepressant drugs showed agomelatine to be one of the most effective and one of only two medications found to be more tolerable than placebo.[14]
A meta-analysis found that agomelatine is effective in treating severe depression. Its antidepressant effect is greater for more severe depression. In people with a greater baseline score (>30 on HAMD17 scale), the agomelatine-placebo difference was of 4.53 points.[15] Controlled studies in humans have shown that agomelatine is at least as effective as the SSRI antidepressants paroxetine, sertraline, escitalopram, and fluoxetine in the treatment of major depression.[16] A 2018 meta-study comparing 21 antidepressants found agomelatine was one of the more tolerable, yet effective antidepressants.[8]
However, the body of research on agomelatine has been substantially affected by publication bias, prompting analyses which take into account both published and unpublished studies.[7][17][18] These have confirmed that agomelatine is approximately as effective as more commonly used antidepressants (e.g. SSRIs), but some qualified this as "marginally clinically relevant",[18] being only slightly above placebo.[17][18] According to a 2013 review, agomelatine did not seem to provide an advantage in efficacy over other antidepressants for the acute-phase treatment of major depression.[19]
Generalized anxiety disorder
Agomelatine has been found more effective than placebo in the treatment of generalized anxiety disorder in a number of short-term double-blind placebo-controlled studies and in long term relapse prevention.[20][21][22][23][24]
Use in special populations
It is not recommended in Europe for use in children and adolescents below 18 years of age due to a lack of data on safety and efficacy.[12] However, a recent 12 week study first reported in September 2020, and published in 2022 showed greater efficacy vs. placebo for agomelatine 25 mg per day in youth age 7–17 years and an acceptable tolerability profile with similar efficacy to fluoxetine.[25][26] Only limited data is available on use in elderly people ≥ 75 years old with major depressive episodes.[12]
It is not recommended during pregnancy or breastfeeding.[9]
Contraindications
Agomelatine is contraindicated in patients with kidney or liver impairment.[12] According to information disclosed by Servier in 2012, guidelines for the follow-up of patients treated with Valdoxan have been modified in concert with the European Medicines Agency. As some patients may experience increased levels of liver enzymes in their blood during treatment with Valdoxan, doctors have to run laboratory tests to check that the liver is working properly at the initiation of the treatment and then periodically during treatment, and subsequently decide whether to pursue the treatment or not.[27] No relevant modification in agomelatine pharmacokinetic parameters in patients with severe renal impairment has been observed. However, only limited clinical data on its use in depressed patients with severe or moderate renal impairment with major depressive episodes is available. Therefore, caution should be exercised when prescribing agomelatine to these patients.[12]
Adverse effects
Agomelatine does not alter daytime vigilance and memory in healthy volunteers. In depressed patients, treatment with the drug increased slow wave sleep without modification of REM (rapid eye movement) sleep amount or REM latency.[28] Agomelatine also induced an advance of the time of sleep onset and of minimum heart rate. From the first week of treatment, onset of sleep and the quality of sleep were significantly improved without daytime clumsiness as assessed by patients.[2][12]
Agomelatine appears to cause fewer sexual side effects and discontinuation effects than paroxetine.[2]
- Hyperhidrosis (excess sweating that is not proportionate to the ambient temperature)
- Abdominal pain
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Back pain
- Fatigue
- Increased ALAT and ASAT (liver enzymes)
- Headache
- Dizziness
- Somnolence
- Insomnia
- Migraine
- Anxiety
- Paraesthesia (abnormal sensations [e.g. itching, burning, tingling, etc.] due to malfunctioning of the peripheral nerves)
- Blurred vision
- Eczema
- Pruritus (itching)
- Urticaria
- Agitation
- Irritability
- Restlessness
- Aggression
- Nightmares
- Abnormal dreams
- Mania
- Hypomania
- Suicidal ideation
- Suicidal behaviour
- Hallucinations
- Steatohepatitis
- Increased GGT and/or alkaline phosphatase
- Liver failure
- Jaundice
- Erythematous rash
- Face oedema and angioedema
- Weight gain or loss, which tends to be less significant than with SSRIs[31]
Dependence and withdrawal
No dosage tapering is needed on treatment discontinuation.[12] Agomelatine has no abuse potential as measured in healthy volunteer studies.[2][12]
Overdose
Agomelatine is expected to be relatively safe in overdose.[32]
Interactions
Agomelatine is a substrate of CYP1A2, CYP2C9 and CYP2C19. Inhibitors of these enzymes, e.g. the SSRI antidepressant fluvoxamine, reduce its clearance and can lead to an increase in agomelatine exposure, and possibly serotonin syndrome .[2][29] There is also the potential for agomelatine to interact with alcohol to increase the risk of hepatotoxicity.[2][29]
Pharmacology
Pharmacodynamics
Agomelatine is a melatonin receptor agonist (MT1 (Ki 0.1 nM) and MT2 (Ki = 0.12 nM)) and serotonin 5-HT2C (Ki = 631 nM) and 5-HT2B receptor (Ki = 660 nM) antagonist.[33][34] Binding studies indicate that it has no effect on monoamine uptake and no affinity for adrenergic, histamine, cholinergic, dopamine, and benzodiazepine receptors, nor other serotonin receptors.[12]
Agomelatine resynchronizes circadian rhythms in animal models of delayed sleep phase syndrome.[35] By antagonizing 5-HT2C, it disinhibits/increases noradrenaline and dopamine release specifically in the frontal cortex. Therefore, it is sometimes classified as a norepinephrine–dopamine disinhibitor. However, the clinical significance of 5-HT2C antagonism by agomelatine is disputed.[36] Unlike clinically significant 5-HT2C antagonists, clinically relevant doses of agomelatine fail to acutely increase slow wave sleep in humans.[37][38]
Agomelatine has shown an antidepressant-like effect in animal models of depression (learned helplessness test, despair test, chronic mild stress) as well as in models with circadian rhythm desynchronisation and in models related to stress and anxiety. In humans, agomelatine has positive phase-shifting properties; it induces a phase advance of sleep, body temperature decline, and melatonin onset.[12]
Chemistry
Structure
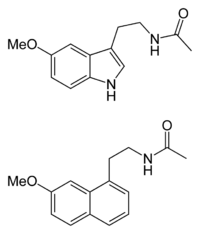
The chemical structure of agomelatine is very similar to that of melatonin. Where melatonin has an indole ring system, agomelatine has a naphthalene bioisostere instead.[39]
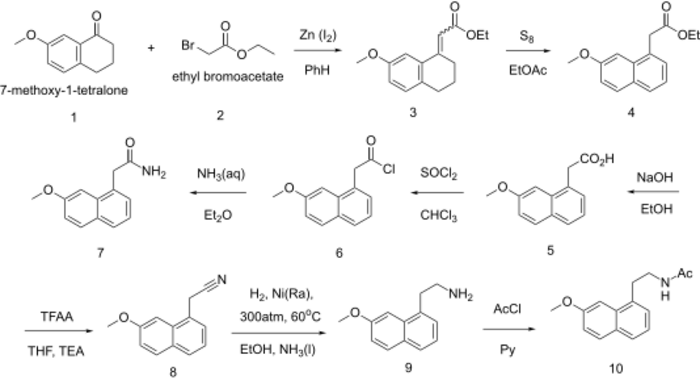
Synthesis
History
Agomelatine was discovered and developed by the European pharmaceutical company Servier Laboratories Ltd. Servier continued to develop the drug and conduct phase III trials in the European Union.
In March 2005, Servier submitted agomelatine to the European Medicines Agency (EMA) under the trade names Valdoxan and Thymanax.[44] On 27 July 2006, the Committee for Medical Products for Human Use (CHMP) of the EMA recommended a refusal of the marketing authorisation. The major concern was that efficacy had not been sufficiently shown, while there were no special concerns about side effects.[44] In September 2007, Servier submitted a new marketing application to the EMA.[45]
In March 2006, Servier announced it had sold the rights to market agomelatine in the United States to Novartis.[46] It was undergoing several phase III clinical trials in the US, and until October 2011 Novartis listed the drug as scheduled for submission to the FDA no earlier than 2012.[47] However, the development for the US market was discontinued in October 2011, when the results from the last of those trials became available.[48]
It received approval from the European Medicines Agency (EMA) for marketing in the European Union in February 2009[12] and approval from the Therapeutic Goods Administration (TGA) for marketing in Australia in August 2010.[2]
Research
Circadian rhythm sleep disorders
Agomelatine has been investigated for its effects on sleep regulation due its actions as a melatonin receptor agonist.[49] Studies report various improvements in general quality of sleep metrics, as well as benefits in circadian rhythm sleep disorders.[49][7][35][50] However, research is very limited (e.g., case reports) and agomelatine is not approved for use in the treatment of sleep disorders.[49]
Seasonal affective disorder
A 2019 Cochrane review suggested no recommendations of agomelatine in support of, or against, its use to treat individuals with seasonal affective disorder.[51]
References
- ↑ Kim, HK; Yang, KI (December 2022). "Melatonin and melatonergic drugs in sleep disorders.". Translational and Clinical Pharmacology 30 (4): 163–171. doi:10.12793/tcp.2022.30.e21. PMID 36632077.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 "Valdoxan Product Information" (PDF). TGA eBusiness Services. Servier Laboratories Pty Ltd.. 2013-09-23. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-07273-3.
- ↑ "Valdoxan 25 mg film-coated tablets - Summary of Product Characteristics (SmPC)". 13 July 2020. https://www.medicines.org.uk/emc/product/6564/smpc.
- ↑ "Thymanax EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/thymanax.
- ↑ "Valdoxan EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/valdoxan.
- ↑ 6.0 6.1 6.2 "Agomelatine versus other antidepressive agents for major depression". The Cochrane Database of Systematic Reviews (12): CD008851. December 2013. doi:10.1002/14651858.CD008851.pub2. PMID 24343836.
- ↑ 7.0 7.1 7.2 7.3 "Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies". BMJ 348: g1888. March 2014. doi:10.1136/bmj.g1888. PMID 24647162.
- ↑ 8.0 8.1 "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis" (in en). Lancet 391 (10128): 1357–1366. April 2018. doi:10.1016/S0140-6736(17)32802-7. PMID 29477251.
- ↑ 9.0 9.1 9.2 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 357–358. ISBN 9780857113382.
- ↑ "Product Information: Valdoxan, INN-agomelatine". European Medicines Agency. 13 November 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000915/WC500046227.pdf.
- ↑ 11.0 11.1 11.2 11.3 11.4 "Agomelatine for depression: expanding the horizons?". Expert Opinion on Pharmacotherapy 20 (6): 647–656. April 2019. doi:10.1080/14656566.2019.1574747. PMID 30759026.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 "Summary of Product Characteristics". European Medicine Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000915/WC500046227.pdf.
- ↑ "Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines". J Psychopharmacol 29 (5): 459–525. May 2015. doi:10.1177/0269881115581093. PMID 25969470. https://kclpure.kcl.ac.uk/ws/files/52545391/PMH_24_3_15_British_Association_forPsychopharmacology_Final_Reconciliation_Draft_2_.docx. Retrieved 2023-01-24.
- ↑ "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet 391 (10128): 1357–1366. April 2018. doi:10.1016/S0140-6736(17)32802-7. PMID 29477251.
- ↑ "Severe depression and antidepressants: focus on a pooled analysis of placebo-controlled studies on agomelatine". International Clinical Psychopharmacology 22 (5): 283–91. September 2007. doi:10.1097/YIC.0b013e3280c56b13. PMID 17690597.
- ↑ "Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal". The International Journal of Neuropsychopharmacology 15 (3): 417–28. April 2012. doi:10.1017/S1461145711001301. PMID 21859514.
- ↑ 17.0 17.1 "Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials". The British Journal of Psychiatry 203 (3): 179–87. September 2013. doi:10.1192/bjp.bp.112.120196. PMID 23999482.
- ↑ 18.0 18.1 18.2 "A benefit-risk assessment of agomelatine in the treatment of major depression". Drug Safety 34 (9): 709–31. September 2011. doi:10.2165/11593960-000000000-00000. PMID 21830835.
- ↑ "Agomelatine versus other antidepressive agents for major depression". The Cochrane Database of Systematic Reviews (12): CD008851. December 2013. doi:10.1002/14651858.CD008851.pub2. PMID 24343836.
- ↑ "Is there a role for agomelatine in the treatment of anxiety disorders?A review of published data". International Journal of Immunopathology and Pharmacology 26 (2): 299–304. 2013. doi:10.1177/039463201302600203. PMID 23755745.
- ↑ "Agomelatine in generalized anxiety disorder: an active comparator and placebo-controlled study". Journal of Clinical Psychiatry 75 (4): 362–8. 2014. doi:10.4088/JCP.13m08433. PMID 24569045.
- ↑ "Efficacy of Agomelatine 25-50 mg for the Treatment of Anxious Symptoms and Functional Impairment in Generalized Anxiety Disorder: A Meta-Analysis of Three Placebo-Controlled Studies". Advances in Therapy 38 (3): 1567–1583. 2021. doi:10.1007/s12325-020-01583-9. PMID 33537871.
- ↑ "Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study". Journal of Clinical Psychopharmacology 28 (5): 561–566. 2008. doi:10.1097/JCP.0b013e318184ff5b. PMID 18794654.
- ↑ "Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: A 12-week, double-blind, placebo-controlled study". European Neuropsychopharmacology 27 (5): 526–537. 2017. doi:10.1097/JCP.0b013e318184ff5b. PMID 28298261.
- ↑ "Safety and efficacy of agomelatine in children and adolescents with major depressive disorder receiving psychosocial counselling: a double-blind, randomised, controlled, phase 3 trial in nine countries". Lancet Psychiatry 9 (2): 113–124. 2022. doi:10.1016/S2215-0366(21)00390-4. PMID 34919834.
- ↑ "Agomelatine Effective for Children, Adolescents With Depression" (in en). https://dgnews.docguide.com/agomelatine-effective-children-adolescents-depression.
- ↑ "Information about Valdoxan for patients". Servier. http://www.servier.com/content/information-about-valdoxan-patients.
- ↑ "Major depressive disorder, sleep EEG and agomelatine: an open-label study". The International Journal of Neuropsychopharmacology 10 (5): 691–696. October 2007. doi:10.1017/S1461145707007754. PMID 17477886.
- ↑ 29.0 29.1 29.2 29.3 29.4 Australian Medicines Handbook 2013. Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 9780980579093. https://books.google.com/books?id=tn3-swEACAAJ.
- ↑ 30.0 30.1 30.2 Joint Formulary Committee and Royal Pharmaceutical Society of Great Britain (2013). British National Formulary (BNF) 65. London, UK: Pharmaceutical Press. p. 253. ISBN 978-0857110848. https://books.google.com/books?id=fZLvtHELVdMC. Retrieved 2018-07-24.
- ↑ "Agomelatine in the treatment of major depressive disorder: potential for clinical effectiveness". CNS Drugs 24 (6): 479–99. June 2010. doi:10.2165/11534420-000000000-00000. PMID 20192279.
- ↑ The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. 2012. ISBN 978-0-470-97948-8. https://books.google.com/books?id=ZjEDDQAAQBAJ. Retrieved 2018-07-24.
- ↑ "Depression: chronophysiology and chronotherapy". Biological Rhythm Research 45: 77–91. June 2013. doi:10.1080/09291016.2013.797657.
- ↑ "The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways". The Journal of Pharmacology and Experimental Therapeutics 306 (3): 954–64. September 2003. doi:10.1124/jpet.103.051797. PMID 12750432.
- ↑ 35.0 35.1 "Agomelatine, an innovative pharmacological response to unmet needs". Journal of Psychopharmacology 22 (7 Suppl): 4–8. September 2008. doi:10.1177/0269881108092593. PMID 18753276.
- ↑ "In response to "The effect of agomelatine on 5HT2C receptors in humans: a clinically relevant mechanism?" by Trevor Norman" (in en). Psychopharmacology 221 (1): 179. May 2012. doi:10.1007/s00213-012-2659-3. ISSN 1432-2072.
- ↑ "The effect of agomelatine on 5HT(2C) receptors in humans: a clinically relevant mechanism?". Psychopharmacology 221 (1): 177–8; author reply 179. May 2012. doi:10.1007/s00213-012-2656-6. PMID 22349274.
- ↑ "Major depressive disorder, sleep EEG and agomelatine: an open-label study". The International Journal of Neuropsychopharmacology 10 (5): 691–696. October 2007. doi:10.1017/S1461145707007754. PMID 17477886.
- ↑ "N-[2-(7-Methoxy-1-naphthyl)ethyl]acetamide, a potent melatonin analog". Acta Crystallogr. C 50 (6): 907–910. 1994. doi:10.1107/S0108270193012922. Bibcode: 1994AcCrC..50..907T.
- ↑ Andrieux J, Houssin R, Yous S, Guardiola B, Lesieur D, "Naphthalene derivatives, procedure for their preparation and pharmaceutical compositions containing them.", EP patent application 447285, published 1991-09-18, assigned to Adir
- ↑ Andrieux J, Houssin R, Yous S, Guardiola B, Lesieur D, "Compounds having a naphthalene structure", US patent granted 5225442, issued 6 July 1993, assigned to Adir
- ↑ "Novel naphthalenic ligands with high affinity for the melatonin receptor". Journal of Medicinal Chemistry 35 (8): 1484–6. April 1992. doi:10.1021/jm00086a018. PMID 1315395.
- ↑ "Synthesis and structure-activity relationships of novel naphthalenic and bioisosteric related amidic derivatives as melatonin receptor ligands". Journal of Medicinal Chemistry 37 (20): 3231–9. September 1994. doi:10.1021/jm00046a006. PMID 7932550.
- ↑ 44.0 44.1 "Questions and Answers on Recommendation for Refusal of Marketing Authorisation". European Medicines Agency. 18 November 2006. http://www.emea.europa.eu/humandocs/PDFs/EPAR/valdoxan/H-656-657-RQ&A-en.pdf.
- ↑ "CHMP Assessment Report for Valdoxan". European Medicines Agency. 20 November 2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000915/WC500046226.pdf.
- ↑ "Servier and Novartis sign licensing agreement for agomelatine, a novel treatment for depression". Servier UK. 2006-03-29. http://www.servier.co.uk/news/news-details.asp?StoryID=76.
- ↑ "Clinical trials for agomelatine". ClinicalTrials.gov. National Institutes of Health. http://clinicaltrials.gov/ct2/results?term=agomelatine.
- ↑ "Novartis drops future blockbuster agomelatine.". Scrip Intelligence. 25 October 2011. http://www.scripintelligence.com/home/Novartis-drops-future-blockbuster-agomelatine-322880.
- ↑ 49.0 49.1 49.2 "Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders". Pharmacotherapy 36 (9): 1028–41. September 2016. doi:10.1002/phar.1822. PMID 27500861.
- ↑ "Valdoxan: A New Approach to The Treatment of Depression". Medical News Today (MediLexicon International Ltd.). 2005-04-05. http://www.medicalnewstoday.com/articles/22334.php.
- ↑ "Melatonin and agomelatine for preventing seasonal affective disorder". The Cochrane Database of Systematic Reviews 2019 (6): CD011271. June 2019. doi:10.1002/14651858.CD011271.pub3. PMID 31206585.
External links
- Genf interaction table- https://www.hug.ch/sites/interhug/files/structures/pharmacologie_et_toxicologie_cliniques/carte_cytochromes_2016_final.pdf
 |