Chemistry:25B-NBOMe
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
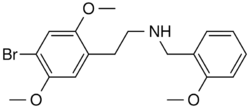
| Formula | C18H22BrNO3 |
| Molar mass | 380.282 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
25B-NBOMe (NBOMe-2C-B, Cimbi-36, Nova, BOM 2-CB) is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor.[2][3][4][5][6][excessive citations] Anecdotal reports from users[weasel words] suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 250–500 µg,[citation needed] making it a similar potency to other phenethylamine derived hallucinogens such as Bromo-DragonFLY. Duration of effects lasts about 12–16 hours[citation needed], although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses.[6] Recently, Custodio et al (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.[7]
The carbon-11 labeled version of this compound ([11C]Cimbi-36) was synthesized and validated as a radioactive tracer for positron emission tomography (PET) in Copenhagen.[8][9][10][11][12][excessive citations] As a 5-HT2A receptor agonist PET radioligand, [11C]Cimbi-36 was hypothesized to provide a more functional marker of these receptors. Also, [11C]Cimbi-36 is investigated as a potential marker of serotonin release and thus could serve as an indicator of serotonin levels in vivo. [11C]Cimbi-36 is now undergoing clinical trials as a PET-ligand in humans.[13][14][15]
Toxicity and harm potential
Neurotoxic and cardiotoxic actions
Emergency treatment
Analogues and derivatives
Analogues and derivatives of 2C-B:
- 25B-NB3OMe
- 25B-NB4OMe
- 25B-NBF
- 25B-NBMD
- 25B-NBOH
- 25B-NBOMe (NBOMe-2CB)
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
Other:
- 2C-B-AN
- 2C-B-BUTTERFLY
- 2C-B-DragonFLY
- 2CB-5-hemifly
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- TCB-2 (2C-BCB)
Legal status
Canada
As of October 31, 2016; 25B-NBOMe is a controlled substance (Schedule III) in Canada.[16]
Russia
Banned as a narcotic drug since May 5, 2015.[17]
Sweden
In Sweden, the Riksdag added 25B-NBOMe to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of August 1, 2013, published by the Medical Products Agency in their regulation LVFS 2013:15 listed as 25B-NBOMe 2-(4-bromo-2,5-dimetoxifenyl)-N-(2-metoxibensyl)etanamin.[18]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[19]
United States
In November 2013, the U.S. Drug Enforcement Administration placed 25B-NBOMe (along with 25I-NBOMe and 25C-NBOMe) in Schedule I of the Controlled Substances Act, making it illegal to manufacture, buy, possess, process, or distribute.[20]
China
As of October 2015 25B-NBOMe is a controlled substance in China.[21]
Czech Republic
25B-NBOMe is banned in the Czech Republic.[22]
Notes
References
- ↑ "Substance Details 25B-NBOMe". https://www.unodc.org/LSS/Substance/Details/8a781e2b-b49a-4baa-b71f-ad1124864662.
- ↑ "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts." (in de). diss.fu-berlin.de. February 28, 2010. http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221.
- ↑ Silva M (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (Ph.D. thesis). Universität Regensburg.
- ↑ "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design 25 (1): 51–66. January 2011. doi:10.1007/s10822-010-9400-2. PMID 21088982. Bibcode: 2011JCAMD..25...51S.
- ↑ "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience 5 (3): 243–9. March 2014. doi:10.1021/cn400216u. PMID 24397362.
- ↑ 6.0 6.1 "Serotonin 2A receptor agonist binding in the human brain with [¹¹CCimbi-36"]. Journal of Cerebral Blood Flow and Metabolism 34 (7): 1188–96. July 2014. doi:10.1038/jcbfm.2014.68. PMID 24780897.
- ↑ "25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential". Addiction Biology 25 (6): e12850. November 2020. doi:10.1111/adb.12850. PMID 31749223.
- ↑ Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- ↑ "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging 38 (4): 681–93. April 2011. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- ↑ "Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [ 11C]Cimbi-36". Molecular Imaging and Biology 15 (4): 376–383. August 2013. doi:10.1007/s11307-012-0609-4. PMID 23306971.
- ↑ "The importance of small polar radiometabolites in molecular neuroimaging: A PET study with [11CCimbi-36 labeled in two positions"]. Journal of Cerebral Blood Flow and Metabolism 38 (4): 659–668. April 2018. doi:10.1177/0271678X17746179. PMID 29215308.
- ↑ "Human biodistribution and radiation dosimetry of the 5-HT2A receptor agonist Cimbi-36 labeled with carbon-11 in two positions". EJNMMI Research 9 (1): 71. July 2019. doi:10.1186/s13550-019-0527-4. PMID 31367837.
- ↑ "From molecule to man: The full CIMBI-36 story". cimbi.dk. http://www.cimbi.dk/images/stories/files/The_full_Cimbi36_story.pdf.
- ↑ "Imanova announces the launch of a new imaging biomarker to investigate the serotonin system in psychiatric illness". imanova.co.uk. http://www.imanova.co.uk/news-and-publications/imanova-news/entry/imanova-announces-the-launch-of-a-new-imaging-biomarker-to-investigate-the-serotonin-system-in-psychiatric-illness.html.
- ↑ "Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels". Neuropsychopharmacology 44 (7): 1328–1334. June 2019. doi:10.1038/s41386-019-0324-9. PMID 30685771.
- ↑ "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Canada Gazette 150 (9). 4 May 2016. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php.
- ↑ "Постановление Правительства РФ от 30.06.1998 N 681 "Об утверждении перечня наркотических средств, психотропных веществ и их прекурсоров, подлежащих контролю в Российской Федерации" (с изменениями и дополнениями)". http://base.garant.ru/12112176/.
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika;" (in sv). lakemedelsverket.se. http://www.lakemedelsverket.se/upload/lvfs/LVFS_2013-15.pdf.
- ↑ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014" (in en). http://www.legislation.gov.uk/uksi/2014/1106/made.
- ↑ "2016 - Final Rule: Placement of Three Synthetic Phenethylamines Into Schedule I" (in en-US). https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr0927_2.htm.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (in cs). Ministerstvo zdravotnictví. http://www.mzcr.cz/Admin/_upload/files/3/Nov%C3%A9%20PL.pdf.
 |

