Chemistry:AB-CHMINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C20H28N4O2 |
| Molar mass | 356.470 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
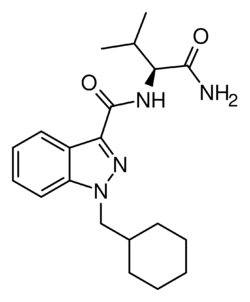
AB-CHMINACA is an indazole-based synthetic cannabinoid. It is a potent agonist of the CB1 receptor (Ki = 0.78 nM) and CB2 receptor (Ki = 0.45 nM) and fully substitutes for Δ9-THC in rat discrimination studies, while being 16x more potent.[2][3] Continuing the trend seen in other cannabinoids of this generation, such as AB-FUBINACA and AB-PINACA, it contains a valine amino acid amide residue as part of its structure, where older cannabinoids contained a naphthyl or adamantane residue.
Side effects
There have been a number of reported cases of seizures, deaths, and psychotic episodes in relation to this synthetic cannabinoid.[4][5][6][7][8][9][10]
Legal status
In 2015, AB-CHMINACA became a Schedule I controlled substance in the United States.[11]
AB-CHMINACA is an Anlage II controlled substance in Germany as of May 2015.[12]
As of October 2015 AB-CHMINACA is a controlled substance in China.[13]
AB-CHMINACA is illegal in Switzerland as of December 2015.[14]
AB-CHMINACA is an illegal substance in Russian Federation.
See also
- 5F-AB-PINACA
- 5F-ADB
- 5F-AMB
- A-CHMINACA[15]
- AB-FUBINACA
- AB-CHFUPYCA
- AB-PINACA
- ADB-CHMINACA
- ADB-FUBINACA
- ADB-PINACA
- ADBICA
- APICA
- APINACA
- MDMB-CHMICA
- MDMB-CHMINACA
- MDMB-FUBINACA
- PX-3
References
- ↑ "Substance Details AB-CHMINACA". https://www.unodc.org/LSS/Substance/Details/45f2f4f5-3c8b-40f3-99d3-207d2666adc2.
- ↑ "AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Δ9-Tetrahydrocannabinol-Like Effects in Mice". The Journal of Pharmacology and Experimental Therapeutics 354 (3): 328–39. September 2015. doi:10.1124/jpet.115.225326. PMID 26105953. PMC 4538877. http://jpet.aspetjournals.org/content/early/2015/06/23/jpet.115.225326.short.
- ↑ AB-CHMINACA, Cayman Chemicals
- ↑ "N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (AB-CHMINACA), N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and [1-(5-fluoropentyl)-1H-indazol-3-yl(naphthalen-1-yl)methanone (THJ-2201). Background Information and Evaluation of 'Three Factor Analysis' (Factors 4, 5, and 6) for Temporary Scheduling."]. Drug Enforcement Administration. December 2014. https://www.grassley.senate.gov/sites/default/files/news/upload/3-factor%20analysis%20AB-CHMINACA%20AB-PINACA%20THJ2201%2012172014.pdf.
- ↑ "Vertex: Police warn of 'ticking time bomb' of potentially lethal cannabis substitute". The Independent. 5 June 2015. https://www.independent.co.uk/news/uk/home-news/vertex-police-warn-of-ticking-time-bomb-of-potentially-lethal-cannabis-substitute-10301030.html.
- ↑ "Synthetic Cannabinoid-Related Illnesses and Deaths". The New England Journal of Medicine 373 (2): 103–7. July 2015. doi:10.1056/NEJMp1505328. PMID 26154784.
- ↑ "Identification and quantification of metabolites of AB-CHMINACA in a urine specimen of an abuser". Legal Medicine 19: 113–8. March 2016. doi:10.1016/j.legalmed.2015.07.011. PMID 26257317. http://www.legalmedicinejournal.com/article/S1344-6223%2815%2930011-0/abstract.
- ↑ "No criminal charges in March death of Terrance Moxley". Richland Source. 30 September 2015. http://www.richlandsource.com/news/no-criminal-charges-in-march-death-of-terrance-moxley/article_4a8029ea-67b9-11e5-a0f0-631f701f77ae.html.
- ↑ "An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA". Clinical Toxicology 53 (10): 950–6. November 2015. doi:10.3109/15563650.2015.1100306. PMID 26555732.
- ↑ "Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC". Forensic Science International 265: 121–4. August 2016. doi:10.1016/j.forsciint.2016.01.018. PMID 26890319.
- ↑ Drug Enforcement Administration, Department of Justice (January 2015). "Schedules of controlled substances: temporary placement of three synthetic cannabinoids into schedule I. Final order". Federal Register 80 (20): 5042–7. PMID 25730924. https://www.gpo.gov/fdsys/pkg/FR-2015-01-30/pdf/2015-01776.pdf.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)". http://www.gesetze-im-internet.de/btmg_1981/anlage_ii.html.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien". Der Bundesrat. https://www.admin.ch/opc/de/classified-compilation/20101220/index.html.
- ↑ "in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA". ACS Chemical Neuroscience 11 (24): 4434–4446. November 2020. doi:10.1021/acschemneuro.0c00644. PMID 33253529.
 |

