Chemistry:XLR-11
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C21H28FNO |
| Molar mass | 329.459 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
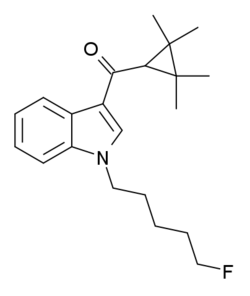
XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively.[1] It is a 3-(tetramethylcyclopropylmethanoyl)indole derivative related to compounds such as UR-144, A-796,260 and A-834,735, but it is not specifically listed in the patent or scientific literature alongside these other similar compounds,[2][3] and appears to have not previously been made by Abbott Laboratories, despite falling within the claims of patent WO 2006/069196. XLR-11 was found to produce rapid, short-lived hypothermic effects in rats at doses of 3 mg/kg and 10 mg/kg, suggesting that it is of comparable potency to APICA and STS-135.[1]
Detection
A forensic standard for this compound is available, and a representative mass spectrum has been posted on Forendex.[4]
Recreational use
XLR-11 was instead first identified by laboratories in 2012 as an ingredient in synthetic cannabis smoking blends, and appears to be a novel compound invented specifically for grey-market recreational use.[5]
Legal Status
XLR-11 was banned in New Zealand by being added to the temporary class drug schedule, effective from 13 July 2012.[6]
The U.S. Drug Enforcement Administration (DEA) made XLR11 illegal under the Federal Controlled Substances act for the foreseeable future as of January 2024.[7]
It has also been banned in Florida as of 11 December 2012.[8]
Arizona banned XLR-11 on 3 April 2013.[9]
As of October 2015 XLR-11 is a controlled substance in China.[10]
XLR-11 is banned in the Czech Republic.[11]
Side effects
XLR-11 has been linked to hospitalizations due to its use.[12]
Toxicity
XLR-11 has been linked to acute kidney injury in some users,[13] along with AM-2201.[14][15]
See also
References
- ↑ 1.0 1.1 "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience 6 (8): 1445–1458. August 2015. doi:10.1021/acschemneuro.5b00107. PMID 25921407. https://zenodo.org/record/47750.
- ↑ Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", WO patent application 2006069196, published 2006-06-29, assigned to Abbott Laboratories
- ↑ "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry 53 (1): 295–315. January 2010. doi:10.1021/jm901214q. PMID 19921781.
- ↑ "XLR-11". Structural, chemical, and analytical data on controlled substances. Southern Association of Forensic Scientists (SAFS). http://forendex.southernforensic.org/index.php/detail/index/1219%20XLR-11.
- ↑ "Bioisosteric Fluorine in the Clandestine Design of Synthetic Cannabinoids". Australian Journal of Chemistry 68: 4. 2015. doi:10.1071/CH14198.
- ↑ "CB-13, MAM-2201, AKB48, and XLR11 are classified as temporary class drugs". Temporary Class Drug Notice. The Department of Internal Affairs: New Zealand Gazette. 2012-07-05. http://www.dia.govt.nz/MSOS118/On-Line/NZGazette.nsf/6cee7698a9bbc7cfcc256d510059ed0b/1800482ea20e00f8cc257a32005ae9f8!OpenDocument.
- ↑ DEA. "Error: no
|title=specified when using {{Cite web}}". https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf. - ↑ "Attorney General Pam Bondi Outlaws Additional Synthetic Drugs" (Press release). State of Florida. 2012-12-11.
- ↑ "Governor Jan Brewer Signs Legislation to Combat Production, Use of Dangerous Drugs" (PDF) (Press release). Office of the Governor, State of Arizona. Archived from the original (PDF) on 7 June 2013. Retrieved 2014-08-27.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (in cs). Ministerstvo zdravotnictví. http://www.mzcr.cz/Admin/_upload/files/3/Nov%C3%A9%20PL.pdf.
- ↑ "Synthetic Cannabinoid-Related Illnesses and Deaths". The New England Journal of Medicine 373 (2): 103–107. July 2015. doi:10.1056/NEJMp1505328. PMID 26154784.
- ↑ "Alphabet Soup, or the newer synthetic cannabinoids...". The Dose Makes The Poison Blog. 11 December 2013. http://dosemakespoison.blogspot.co.uk/2013/12/alphabet-soup-or-newer-synthetic.html.
- ↑ "AKI associated with synthetic cannabinoids: a case series". Clinical Journal of the American Society of Nephrology 8 (4): 523–526. April 2013. doi:10.2215/CJN.05690612. PMID 23243266.
- ↑ "Acute Kidney Injury Associated with Synthetic Cannabinoid Use – Multiple States, 2012". Morbidity and Mortality Weekly Report. U.S. Centers for Disease Control and Prevention (CDC). 2013-02-15. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6206a1.htm?s_cid=mm6206a1_x.
 |

