Chemistry:AM404
This article is missing information about history of discovery — who gave it this codename?. (December 2022) |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
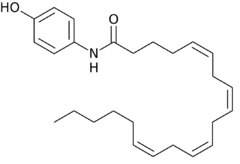
| Formula | C26H37NO2 |
| Molar mass | 395.587 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM404, also known as N-arachidonoylphenolamine,[1][2] is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action[3] and anticonvulsant effects.[4] Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.
Pharmacology
It is established that AM404 increases concentrations of the endogenous cannabinoid anandamide within the synaptic cleft, contributing to its analgesic activity.[5] This has been well characterised as involving endocannabinoid transporter inhibition, but the precise transporter responsible is yet to be determined.[5][6][7]
AM404 was originally reported to be an endogenous cannabinoid reuptake inhibitor, preventing the transport of anandamide and other related compounds back from the synaptic cleft, much in the same way that common selective serotonin reuptake inhibitor (SSRI) antidepressants prevent the reuptake of serotonin. Earlier work on the mechanism of AM404 suggested that the inhibition of fatty acid amide hydrolase (FAAH) by AM404 was responsible for all of its attributed reuptake properties, since intracellular FAAH hydrolysis of anandamide changes the intra/extracellular anandamide equilibrium.[7] However, this is not the case, as newer research on FAAH knockout mice has found that brain cells internalize anandamide through a selective transport mechanism which is independent of FAAH activity.[6] It is this mechanism which is inhibited by AM404.
AM404 is also a TRPV1 agonist and inhibitor of cyclooxygenase COX-1 and COX-2, thus attenuating prostaglandin synthesis. AM404 is thought to induce its analgesic action through its activity on the endocannabinoid, COX, and TRPV systems, all of which are present in pain and thermoregulatory pathways.[5] AM404 activates vanilloid receptors causing vasodilation which is inhibited by the vanilloid receptor antagonist capsazepine.[8]
The anticonvulsant action is mediated through CB1 receptors.[4]
See also
- VDM-11 (2-methyl analogue)
References
- ↑ "Oral acetaminophen-induced spinal 5-hydroxytriyptamine release produces analgesic effects in the rat formalin test". Biomedicine & Pharmacotherapy 146: 112578. February 2022. doi:10.1016/j.biopha.2021.112578. PMID 34959121.
- ↑ "Novel bioactive metabolites of dipyrone (metamizol)". Bioorganic & Medicinal Chemistry 20 (1): 101–107. January 2012. doi:10.1016/j.bmc.2011.11.028. PMID 22172309.
- ↑ "The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors". European Journal of Pharmacology 531 (1–3): 280–281. February 2006. doi:10.1016/j.ejphar.2005.12.015. PMID 16438952.
- ↑ 4.0 4.1 "Acetaminophen inhibits status epilepticus in cultured hippocampal neurons". NeuroReport 22 (1): 15–18. January 2011. doi:10.1097/WNR.0b013e3283413231. PMID 21037491.
- ↑ 5.0 5.1 5.2 "Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system". The Journal of Biological Chemistry 280 (36): 31405–31412. September 2005. doi:10.1074/jbc.M501489200. PMID 15987694.
- ↑ 6.0 6.1 "Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172". Proceedings of the National Academy of Sciences of the United States of America 101 (23): 8756–8761. June 2004. doi:10.1073/pnas.0400997101. PMID 15138300.
- ↑ 7.0 7.1 "Evidence against the presence of an anandamide transporter". Proceedings of the National Academy of Sciences of the United States of America 100 (7): 4269–4274. April 2003. doi:10.1073/pnas.0730816100. PMID 12655057. Bibcode: 2003PNAS..100.4269G.
- ↑ "The anandamide transport inhibitor AM404 activates vanilloid receptors". European Journal of Pharmacology 396 (1): 39–42. May 2000. doi:10.1016/s0014-2999(00)00207-7. PMID 10822052.
 |

