Chemistry:THC-O-acetate
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C23H32O3 |
| Molar mass | 356.506 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
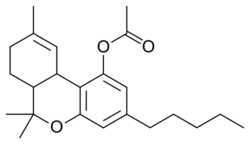
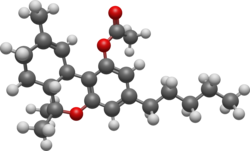
THC-O-acetate (THC acetate ester, O-acetyl-THC, THC-O, AcO-THC) is the acetate ester of THC. The term THC-O-acetate and its variations are commonly used for two types of the substance, dependent on which cannabinoid it is synthesized from. The difference between Δ8-THC and Δ9-THC is bond placement on the cyclohexene ring.[1]
Physical data, chemistry, and properties
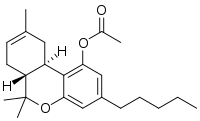
THC acetate ester (THC-O or THCOA) can be synthesized from THC,[2][3] or from THCA. The acetylation of THC does not change the properties of the compound to the same extent as with other acetate esters, as the parent compound (THC) is already highly lipophilic, but potency is nonetheless increased to some extent. While the acetate ester of Δ9-THC is the best studied, the acetate esters of other isomers, especially Δ8-THC but also Δ10-THC are also known, as are other esters such as THC-O-propionate, THC-O-phosphate, THC hemisuccinate, THC hemiglutarate, THC morpholinylbutyrate, THC piperidinylpropionate,[4] and THC naphthoyl ester (THC-NE),[5] as well as the hydrogenated derivative HHC-O-acetate and the ring-expanded abeo-HHC acetate.
Pharmacology
It is a metabolic pro-drug, with its subjective effects being felt around 30 minutes after ingestion.[2][6]
Psychedelic claims
In a 2023 study, anecdotal claims surrounding THC-O-acetate's supposed ability to initiate psychedelic experiences were shown to not be significant. Answers using the Mystical Experience Questionnaire (MEQ) were under the threshold of a true experience, and those who had used classical psychedelics such as LSD or psilocybin consistently scored lower on the MEQ. When asked directly, 79% of the participants said it was either "not at all" or "a little" like a psychedelic experience.[7]
History
THC acetate ester was investigated as a possible non-lethal incapacitating agent as part of the Edgewood Arsenal experiments at some point between 1949 and 1975. It was noted to have about twice the capacity to produce ataxia (lack of voluntary coordination of muscle movements) as did THC when administered to dogs.[8]
Author D. Gold provided synthesis instructions for this compound (calling it "THC acetate") in his 1974 book Cannabis Alchemy: Art of Modern Hashmaking, in which it is described as follows;[2]
"The effect of the acetate is more spiritual and psychedelic than that of the ordinary product. The most unique property of this material is that there is a delay of about thirty minutes before its effects are felt."
The U.S. DEA first encountered THC-O-acetate as an apparent controlled substance analogue of THC in 1978. It was made in an analogous manner to how aspirin (acetylsalicylic acid) is made from willow bark (salicylic acid).[9] The incident was described by Donald A. Cooper of the DEA thus:
"Given the world wide ready availability of marijuana, it is somewhat difficult to produce a viable argument for making [controlled substance analogs (CsA's)] of cannabinoids. However, ten years ago (1978) an attempt to produce CsA's from cannabis extracts was encountered in the Jacksonville, Florida area. In this case a concentrated extract of cannabis had been obtained by a soxhlet extraction. The extract had been acetylated with acetic anhydride, and in the final step, the excess acetic anhydride removed by distillation (reference is unretrievable due to its appearance in an underground periodical). The product contained neither quantities of nonderivatized cannabinoid nor any identifiable plant fragments. Since this single instance, no acetalaced cannabinoid samples have been reported by a DEA laboratory. Therefore, this instance is assumed to represent an isolated occurrence and as such, will serve to terminate our discussion of cannabinoid CsA's."
A similar case reported in June 1995 in the United Kingdom , and THC-O-acetate was ruled to be a Class A drug in that case. The description of that case appears to indicate the convicted manufacturer was using D. Gold's book Cannabis Alchemy as a guide.[10]
THC acetate was also reported to have been found by New Zealand police in 1995, again made by acetylation of purified cannabis extracts with acetic anhydride.[11]
Following legal changes in the United States since around 2018, especially the legalisation of cannabis in an increasing number of states and the passage of the 2018 Farm Bill[12] which eased restrictions on the cultivation of industrial hemp, THC-O-acetate has become increasingly available as a recreational drug, initially in the United States[13] but subsequently in other countries also.[where?] It may be produced from extracts of psychoactive strains of cannabis in states where this is permitted, in which case the product will be primarily O-acetyl-Δ9-THC, but it is also commonly produced from Delta-8-THC which is synthesised from cannabidiol extracted from hemp, in which case the product will be primarily O-acetyl-Δ8-THC. Since Δ9-THC and Δ8-THC are quite different in potency, the corresponding acetylated derivatives also will be, which may pose risks to consumers who are unaware of which isomer is in commercial products.
Concerns have also been raised about the lack of safety data or quality control testing given the limited history of human use and potentially toxic chemical reagents used during manufacture. The legal issue of whether purified THC-O-acetate is considered legal in jurisdictions where cannabis is legal is also complex and varies between jurisdictions.[14][15][16]
Toxicity
In 2022, researchers at Portland State University who screened for the presence of reacted ketene as N-benzylacetamide reported that Vitamin E acetate, CBD-acetate, CBN-acetate and THC-O-acetate may break down to release ketene gas when heated at 340 °C (644 °F).
The lowest concentration of inhaled ketene that produces a physiologically negative response is 5 ppm (5 parts per million).[17] For this reason it is advised to exercise caution around THC-O acetate and other acetate esters of inhaled drugs.
Legal status
United Kingdom
THC-O-acetate is a Class B drug in the United Kingdom .[18]
United States
THC-O-acetate is not directly listed under the Controlled Substances Act, but it is designated as a Schedule I controlled substance in the United States.[19][20] This designation is based upon a private letter ruling by DEA which communicated that THC-O-Acetate met the statutory definition of ‘tetrahydrocannabinol’. DEA reached this conclusion primarily on the basis that THC-O-Acetate is not known to occur in nature. Consequently, THC-O-Acetate cannot be derived from either ‘Hemp’ or ‘Marihuana’ without chemical conversion:[19][21]
“Delta-9-THCO and delta-8-THCO do not occur naturally in the cannabis plant and can only be obtained synthetically, and therefore do not fall under the definition of hemp…Thus, Δ9-THC-O and Δ8-THC-O meet the definition of 'tetrahydrocannabinols,' and they [and products containing Δ9-THC-O and Δ8-THC-O] are controlled in schedule I by 21 U.S.C. § 812(c) Schedule I, and 21 CFR § 1308.11(d).”
This was thought by some to conflict with the statutory definition of hemp.[22] The statutory definition is the following:
"(1) HEMP.—The term 'hemp' means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a Δ9-THC concentration of not more than 0.3 percent on a dry weight basis."
Its sudden status in the early 2020s as both a Schedule I and widely commercially distributed chemical is a heavily debated topic, most notably in the United States.
See also
- Acetylation
- Abeo-HHC acetate
- Cannabidiol diacetate
- Cod-THC
- Nabitan
- O-1057
- O-2694
- HHCP-O-acetate
- THCP-O-acetate
References
- ↑ "Delta-8 And -9 THC-O Are Controlled Substances, DEA Says". February 16, 2023. https://www.forbes.com/sites/dariosabaghi/2023/02/16/delta-8-and9-thc-o-are-controlled-substances-dea-says/?sh=7d5c2068754f.
- ↑ 2.0 2.1 2.2 Cannabis Alchemy: Art of Modern Hashmaking. Ronin Publishing (2010). 1974. ISBN 978-1-5795-1095-4. http://www.kindgreenbuds.com/cannabis-alchemy/thc-acetate/.
- ↑ Marijuana Chemistry: Genetics, Processing, Potency. Ronin Publishing. 1990. ISBN 978-0-9141-7139-3. https://books.google.com/books?id=1YeyucgLHnQC&q=marijuana+chemistry.
- ↑ Banerjee A, et al. “Breaking bud”: the effect of direct chemical modifications of phytocannabinoids on their bioavailability, physiological effects, and therapeutic potential. Org. Biomol. Chem., 2023,21, 3715-3732. doi:10.1039/D3OB00068K
- ↑ Elkarim NS. Stable formulations of dronabinol. Patent US20210251947
- ↑ "Marijuana Potency". And/Or Press. 1977. pp. 174. https://books.google.com/books?id=_tVJAQAAIAAJ.
- ↑ "THC-O-Acetate: Scarce Evidence for a Psychedelic Cannabinoid". Journal of Psychoactive Drugs: 1–5. June 2023. doi:10.1080/02791072.2023.2230573. PMID 37381980.
- ↑ Committee on Toxicology, National Research Council (1984). Possible Long-Term Health Effects of Short-Term Exposure To Chemical Agents, Volume 2: Cholinesterase Reactivators, Psychochemicals and Irritants and Vesicants. The National Academies Press. p. 79. ISBN 978-0-309-07772-9. http://books.nap.edu/openbook.php?record_id=9136&page=79.
- ↑ "Future Synthetic Drugs of Abuse.". Drug Enforcement Administration. McLean, Virginia. https://www.erowid.org/library/books_online/future_synthetic/future_synthetic.shtml.
- ↑ Cannabis: The Genus Cannabis. Hardwood Academic Publishers. 2003. p. 82. ISBN 90-5702-291-5. https://books.google.com/books?id=EgicqjKEowQC&q=pirnabine&pg=PA80.
- ↑ "Δ9-THC acetate from acetylation of cannabis oil.". Science and Justice 36 (3): 195–197. 1995. doi:10.1016/S1355-0306(96)72595-9.
- ↑ "President Trump Signed the Farm Bill into Law One Year Ago Today, USDA Highlights Implementation Accomplishments to Date" (Press release). Washington D.C.: USDA Press. USDA. 2019-12-20. Retrieved 2023-06-22.
- ↑ "∆8-THC, THC-O Acetates and CBD-di-O Acetate: Emerging Synthetic Cannabinoids Found in Commercially Sold Plant Material and Gummy Edibles". Journal of Analytical Toxicology 46 (8): 940–948. October 2022. PMID 35674405.
- ↑ "THC-O-acetate safety concerns: Q&A with scientist James Stephens.". MJBizDaily. 3 August 2021. https://mjbizdaily.com/scientist-explains-safety-concerns-around-thc-o-acetate/.
- ↑ "THC-O Acetate: Everything you need to know about safety, purity and effects.". ACS Laboratory. 5 August 2021. https://acslabcannabis.com/blog/cannabinoids/thc-o-acetate-everything-you-need-to-know-about-safety-purity-and-effects/.
- ↑ "Delta-8-THC craze concerns chemists.". Chemical & Engineering News 99 (31). 30 August 2021. https://cen.acs.org/biological-chemistry/natural-products/Delta-8-THC-craze-concerns/99/i31.
- ↑ Ketene. National Academies Press (US). 21 March 2014. https://www.ncbi.nlm.nih.gov/books/NBK224928/.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2008". Office of Public Sector Information. http://www.legislation.gov.uk/uksi/2008/3130/contents/made.
- ↑ 19.0 19.1 "Delta-8 And -9 THC-O Are Controlled Substances, DEA Says" (in en). https://www.forbes.com/sites/dariosabaghi/2023/02/16/delta-8-and9-thc-o-are-controlled-substances-dea-says/.
- ↑ "Response Letter: Control status under the Controlled Substances Act (CSA) of THC acetate ester (THCO)". U. S. Department of Justice, Drug Enforcement Administration. 13 February 2023. https://cannabusiness.law/wp-content/uploads/DEA-THCO-response-to-Kight.pdf.
- ↑ "Controlled Substances, Alphabetical Order". Orange Book. Diversion Control Division, Drug Enforcement Agency, U.S. Department of Justice. https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf.
- ↑ "7 U.S. Code § 1639o - Definitions" (in en). https://www.law.cornell.edu/uscode/text/7/1639o.
 |