Chemistry:AB-001
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
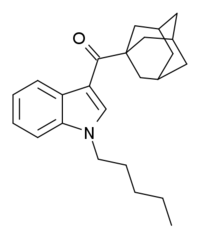
| Formula | C24H31NO |
| Molar mass | 349.518 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AB-001 (1-pentyl-3-(1-adamantoyl)indole) is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011.[1][2][3] It is unclear who AB-001 was originally developed by, but it is structurally related to compounds such as AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are known to be potent cannabinoid agonists with moderate to a high selectivity for CB2 over CB1.[4][5] The first published synthesis and pharmacological evaluation of AB-001 revealed that it acts as a full agonist at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM).[6] However, AB-001 was found to possess only weak cannabimimetic effects in rats at doses up to 30 mg/kg, making it less potent than the carboxamide analogue APICA, which possesses potent cannabimimetic activity at doses of 3 mg/kg.[6]
See also
- A-834,735
- AB-005
- AM-1248
- APICA
- JWH-018
- JWH-250
- RCS-4
- RCS-8
- MN-25
- UR-144
- Structural scheduling of synthetic cannabinoids
- Adamantane
References
- ↑ "Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary". Forensic Science International 214 (1–3): 27–32. January 2012. doi:10.1016/j.forsciint.2011.07.011. PMID 21813254.
- ↑ Research on Head Shop drugs in Dublin: Part 2
- ↑ "The detection of the urinary metabolites of 3-[(adamantan-1-yl)carbonyl]-1-pentylindole (AB-001), a novel cannabimimetic, by gas chromatography-mass spectrometry". Drug Testing and Analysis 4 (6): 519–24. June 2012. doi:10.1002/dta.350. PMID 22102533.
- ↑ US patent 7820144, Makriyannis A, Deng H, "Receptor selective cannabimimetic aminoalkylindoles", granted 2010-10-26
- ↑ "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry 53 (1): 295–315. January 2010. doi:10.1021/jm901214q. PMID 19921781.
- ↑ 6.0 6.1 "The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse". ACS Chemical Neuroscience 4 (7): 1081–92. July 2013. doi:10.1021/cn400035r. PMID 23551277.
 |

