Chemistry:Cannabichromene
| |

| |
| Names | |
|---|---|
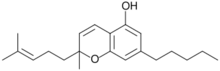
| IUPAC name
2-Methyl-2-(4-methylpent-3-enyl)-7-pentyl-5-chromenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.469 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene,[1] exhibits anti-inflammatory properties in vitro, which may, theoretically, contribute to cannabis analgesic effects.[2] It is a phytocannabinoid, one of the hundreds of cannabinoids found in the Cannabis plant.[3] It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others.[3][4] CBC and cannabinols are present in cannabis.[3] It is not scheduled by the Convention on Psychotropic Substances.
Biosynthesis
Within the Cannabis plant, CBC occurs mainly as cannabichromenic acid (CBCA, 2-COOH-CBC, CBC-COOH). Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (CBGA; the sole intermediate for all other phytocannabinoids), which is cyclized by the enzyme CBCA synthase to form CBCA. Over time, or when heated above 93 °C, CBCA is decarboxylated, producing CBC. See also the biosynthetic scheme image below.[citation needed]
Pharmacology
Cannabichromene has been hypothesized to affect THC psychoactivity, though in vivo effects have not been demonstrated.[5] CBC acts on the TRPV1 and TRPA1 receptors, interfering with their ability to break down endocannabinoids (chemicals such as anandamide and 2-AG that the body creates naturally).[6][unreliable source?] CBC has shown antitumor effects in breast cancer xenoplants in mice.[7] It also has anticonvulsant activity in a mouse model.[8]
In vitro, CBC binds weakly to CB1 and CB2 with binding affinities of 713 nM and 256 nM, respectively, which are significantly lower than that for THC with 35 nM at CB1.[9][10] acting as an agonist for cAMP stimulation and an antagonist at beta-arrestin.[9] Additionally, CBC is an agonist of TRPA1, and less potently TRPV3 and TRPV4.[3] CBC has two stereoisomers.
References
- ↑ "Cannabichromene". National Center for Biotechnology Information. 16 February 2019. https://pubchem.ncbi.nlm.nih.gov/compound/cannabichromene.
- ↑ "Molecular Targets of the Phytocannabinoids: A Complex Picture". Progress in the Chemistry of Organic Natural Products 103: 103–31. 2017. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMID 28120232.
- ↑ 3.0 3.1 3.2 3.3 Turner, Sarah E.; Williams, Claire M.; Iversen, Leslie; Whalley, Benjamin J. (2017). "Molecular Pharmacology of Phytocannabinoids". in Kinghorn, A. Douglas; Falk, Heinz; Gibbons, Simon et al.. Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Progress in the Chemistry of Organic Natural Products. 103. Springer International Publishing. pp. 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6.
- ↑ Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472. https://figshare.com/articles/journal_contribution/5028338.
- ↑ "Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids". Behavioural Pharmacology 16 (5–6): 487–96. September 2005. doi:10.1097/00008877-200509000-00023. PMID 16148455.
- ↑ "What Is CBC (Cannabichromene)?" (in en-US). https://www.cnbs.org/cannabinoids/cbc-cannabichromene/.
- ↑ Ligresti, A.; Moriello, A. S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G. et al. (2006-09-01). "Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma | Journal of Pharmacology and Experimental Therapeutics". Journal of Pharmacology and Experimental Therapeutics 318 (3): 1375–1387. doi:10.1124/jpet.106.105247. PMID 16728591.
- ↑ Anderson LL, Ametovski A, Lin Luo J, Everett-Morgan D, McGregor IS, Banister SD, Arnold JC. Cannabichromene, Related Phytocannabinoids, and 5-Fluoro-cannabichromene Have Anticonvulsant Properties in a Mouse Model of Dravet Syndrome. ACS Chem Neurosci. 2021 Jan 20;12(2):330-339. doi:10.1021/acschemneuro.0c00677 PMID 33395525
- ↑ 9.0 9.1 Zagzoog, Ayat; Mohamed, Kawthar A.; Kim, Hye Ji J.; Kim, Eunhyun D.; Frank, Connor S.; Black, Tallan; Jadhav, Pramodkumar D.; Holbrook, Larry A. et al. (2020-11-23). "In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa" (in en). Scientific Reports 10 (1): 20405. doi:10.1038/s41598-020-77175-y. ISSN 2045-2322. PMID 33230154.
- ↑ Rosenthaler, Sarah; Pöhn, Birgit; Kolmanz, Caroline; Nguyen Huu, Chi; Krewenka, Christopher; Huber, Alexandra; Kranner, Barbara; Rausch, Wolf-Dieter et al. (November 2014). "Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures" (in en). Neurotoxicology and Teratology 46: 49–56. doi:10.1016/j.ntt.2014.09.003. PMID 25311884. https://linkinghub.elsevier.com/retrieve/pii/S089203621400172X.
 |


