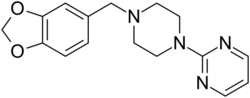
Chemistry:Piribedil
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10% (peak at 1 hour) |
| Protein binding | 70–80% |
| Metabolism | extensive liver |
| Elimination half-life | 1.7–6.9 hours |
| Excretion | Kidney (68%) and bile duct (25%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H18N4O2 |
| Molar mass | 298.346 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Piribedil (trade names Pronoran, Trivastal Retard, Trastal, Trivastan, Clarium and others) is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties.[2][3]
Medical uses
- Treatment of Parkinson's disease (PD), either as monotherapy (without levodopa) or in combination with L-DOPA therapy, in the early stages of the disease as well as in the advanced ones
- Treatment of pathological cognitive deficits in the elderly (impaired attention, motivation, memory, etc.)
- Treatment of dizziness in the young patients
- Treatment of retinal ischemic manifestations
- Adjunctive treatment of intermittent claudication due to peripheral vascular disease (PVD) of the lower limbs (stage 2)
- Adjunctive treatment of anhedonia, apathy and treatment-resistant depression in unipolar and bipolar depressives (off label)
- Treatment of gait disorders associated with Parkinson's disease (no related cause) and other forms of parkinsonism
Other uses
The drug has been shown to enhance working memory capacities in normal aging adults.[4]
In age-related memory impairment, it has a positive effect on psychophysiological state of elderly people, improving memory and attention and increasing the velocity of psychomotor reactions and lability of nervous processes.[5]
It enhances cognitive skill learning in healthy older adults.[6]
It showed a positive effect in restless legs syndrome.[7]
Side effects
- Minor gastrointestinal upset (nausea, vomiting, flatulence, etc.) in predisposed individuals, or when taken between meals: adjust dosage individually, and/or add domperidone;
- Orthostatic hypotension or drowsiness may occur, particularly in predisposed individuals (underlying condition or causative illness);
- Mild dizziness, confusion and feeling "drunk" also may occur.
As with other dopamine agonists (like pramipexole and ropinirole), compulsive behavior like pathological gambling, overeating, excessive shopping, increased libido, sexual and/or other intense urges, may develop.[8][9]
Another rare side effect of piribedil is excessive daytime sleepiness and unintended sleep episodes.[9][10]
Overdose
At very high doses, piribedil has an emetic action on the chemoreceptor trigger zone (CTZ). Tablets will thus be rapidly rejected, which explains why no data are currently available concerning the risk of overdosage.
Interactions
Dopamine antagonists reduce the effect of piribedil.
Pharmacology
Pharmacodynamics
- Dopamine receptor agonist, selective for subtypes D2 and D3.
- Dopamine receptor antagonist, selective for subtypes D4.[11][12][13]
- Adrenergic receptor antagonist, subtypes α2A and α2C.[14]
- Lack of affinity to serotonin receptor 5-HT2B.[14]
References
- ↑ "Active substance: piribedil". List of nationally authorised medicinal products. European Medicines Agency. 26 November 2020. https://www.ema.europa.eu/documents/psusa/piribedil-list-nationally-authorised-medicinal-products-psusa/00002436/202003_en.pdf.
- ↑ "Antiparkinsonian agent piribedil displays antagonist properties at native, rat, and cloned, human alpha(2)-adrenoceptors: cellular and functional characterization". The Journal of Pharmacology and Experimental Therapeutics 297 (3): 876–887. June 2001. PMID 11356907. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11356907.
- ↑ "Piribedil enhances frontocortical and hippocampal release of acetylcholine in freely moving rats by blockade of alpha 2A-adrenoceptors: a dialysis comparison to talipexole and quinelorane in the absence of acetylcholinesterase inhibitors". The Journal of Pharmacology and Experimental Therapeutics 305 (1): 338–346. April 2003. doi:10.1124/jpet.102.046383. PMID 12649387.
- ↑ "Effects of the dopamine agonist piribedil on prefrontal temporal cortical network function in normal aging as assessed by verbal fluency". Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (1): 262–268. January 2007. doi:10.1016/j.pnpbp.2006.06.017. PMID 16876301.
- ↑ "[Efficacy of pronoran in age-related memory impairment]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 105 (2): 46–50. 2005. PMID 15792142.
- ↑ "Cognitive skill learning in healthy older adults after 2 months of double-blind treatment with piribedil". Psychopharmacology 176 (2): 175–181. November 2004. doi:10.1007/s00213-004-1869-8. PMID 15138753.
- ↑ "Piribedil for restless legs syndrome: a pilot study". Movement Disorders 16 (3): 579–581. May 2001. doi:10.1002/mds.1104. PMID 11391766.
- ↑ "Impulse control disorder and piribedil: report of 5 cases". Clinical Neuropharmacology 33 (1): 11–13. 2010. doi:10.1097/WNF.0b013e3181c4ae2e. PMID 19959959.
- ↑ 9.0 9.1 TRIVASTAL Retard 50 (piribedil) Prescribing Information, Servier Laboratories, April 2008. [1]
- ↑ "Piribedil-induced sleep attacks in patients without Parkinson disease: a case series". Clinical Neuropharmacology 34 (3): 104–107. 2011. doi:10.1097/WNF.0b013e31821f0d8b. PMID 21586915.
- ↑ "The selective dopamine D4 receptor antagonist, PNU-101387G, prevents stress-induced cognitive deficits in monkeys". Neuropsychopharmacology 23 (4): 405–10. October 2000. doi:10.1016/S0893-133X(00)00133-0. PMID 10989267.
- ↑ "Randomized study of the dopamine receptor agonist piribedil in the treatment of mild cognitive impairment". The American Journal of Psychiatry 158 (9): 1517–9. September 2001. doi:10.1176/appi.ajp.158.9.1517. PMID 11532743.
- ↑ "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 805–814. November 2002. doi:10.1124/jpet.102.039875. PMID 12388667.
- ↑ 14.0 14.1 "Piribedil" (in de). Neue Arzneimittel 2008.
External links
- "Piribedil". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/piribedil.
 |

