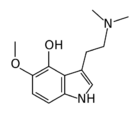
Chemistry:4-Hydroxy-5-methoxydimethyltryptamine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H18N2O2 |
| Molar mass | 234.299 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 146 to 147 °C (295 to 297 °F) (from ethyl acetate[1]) |
| |
| |
| | |
4-Hydroxy-5-methoxydimethyltryptamine, also known as 4-HO-5-MeO-DMT or psilomethoxin, is a hypothetical novel psychedelic drug. It is the 4-hydroxy counterpart of 5-MeO-DMT, or the 5-methoxy counterpart of psilocin.
It is structurally similar to other psychedelic tryptamines, but very little is known about its effects. The only report of it in the chemical literature was a paper published by Marc Julia's group at the Pasteur Institute in 1965.[1] This paper reports a 10 step synthesis of 4-HO-5-MeO-DMT from ortho-vanillin. Alexander Shulgin hypothesized that 4-HO-5-MeO-DMT could be biosynthesized by feeding Psilocybe cultures with 5-MeO-DMT, referencing a 1988 study by Jochen Gartz where transformation of DET into 4-HO-DET and 4-PO-DET was reported using such a method, with neither compounds having ever been found in nature.[2][3]
References
- ↑ 1.0 1.1 "No 209 - Recherches en série indolique. XIV (*) - Sur des méthoxy-5 hydroxy-4, méthoxy-5 hydroxy-6 et méthoxy-7 hydroxy-6 tryptamines" (in fr). Bulletin de la Société Chimique de France: 1417–1423. 1965.
- ↑ "4-Hydroxy-5-methoxy-N,N-dimethyltryptamine, Psilocybe mushrooms, Psilocin" (in en). Ask Dr. Shulgin Online. http://www.cognitiveliberty.org/ccle1/shulgin/blg/2005/12/4-hydroxy-5-methoxy-nn_07.html.
- ↑ "Biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe". Journal of Basic Microbiology 29 (6): 347–352. 1989. doi:10.1002/jobm.3620290608. PMID 2614674.
 |

