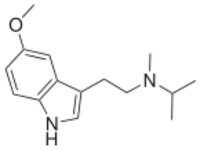
Chemistry:5-MeO-MiPT
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because of the very similar structure and effects.
Chemistry
5-MeO-MiPT is in a class of compounds commonly known as tryptamines, and is the N-methyl-N-isopropyl homologue of the psychedelic, 5-MeO-DMT. The full name of the chemical is 5-methoxy-N-methyl-N-isopropyltryptamine.
5-MeO-MiPT causes the ehrlich reagent to turn purple then fade to faint blue. It causes the marquis reagent to go yellow through to black.[1]
Effects
This is an analogue of the more popular drug 5-MeO-DiPT (nicknamed "foxy methoxy") and has the nickname "moxy". Some users report the tactile effects of 5-MeO-DiPT without some of the unwanted side effects. At higher doses it becomes much more psychedelic sometimes being compared to 5-MeO-DMT. But at doses of 4-10 milligrams users find 5-MeO-MiPT to be a very euphoric and tactile chemical.[2][3] Its energetic effects can be very strong at high doses, increasing normal heart rate considerably. Sounds can be amplified in perception to a point where synesthetic effects ("touching or/and tasting sounds") occur.[4]
Pharmacodynamics
| Binding Sites | Binding Affinity Ki (μM)[5] |
|---|---|
| 5-HT1A | 0.058 |
| 5-HT2A | 0.163 |
| 5-HT2C | 1.3 |
| D1 | >25 |
| D2 | >25 |
| D3 | >25 |
| α1A | >12 |
| α2A | 5.3 |
| TAAR1 | >15 |
| H1 | 3.9 |
| SERT | 3.3 |
| DAT | >26 |
| NET | >22 |
Dosage
Based on many anecdotal reports,[6][7] dosages can be classified as follows:
| Smoked | Oral | |
|---|---|---|
| Threshold | 5 mg | 3 mg |
| Light | 5 - 10 mg | 3 - 7 mg |
| Common | 10 - 15 mg | 7 - 15 mg |
| Strong | 15 - 20 mg | 15 - 20 mg |
| Heavy | 20 mg + | 20 mg + |
Pharmacology
The mechanism that produces the hallucinogenic and entheogenic effects of 5-MeO-MiPT is thought to result primarily from 5-HT2A receptor agonism, although additional mechanisms of action such as inhibition of MAO may be involved also.[8][9] While 5-MeO-MiPT binds most strongly to 5-HT1A receptors, it also shows fairly strong binding affinity to the SERT and NET, thereby acting as a moderately potent serotonin-norepinephrine reuptake inhibitor.[10] These mechanisms may help explain why there are many anecdotal reports of anti-depressant and anxiolytic effects from modest doses of this compound. For example, SNRIs such as venlafaxine are commonly prescribed to treat depression, and the 5-HT1A agonist buspirone is prescribed primarily for treatment of anxiety.
Reagent Results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test. The following test results are from protestkit.
| 5-MeO-MiPT | Marquis | Mecke | Mandelin | Liebermann | Ehrlich | Hofmann | Simon’s |
|---|---|---|---|---|---|---|---|
| Freebase | Orange to brown | Orange red | Deep greenish brown | Unknown | Purple | No reaction | No reaction |
| HCl | Orange to brown | Red to brown | Greenish brown | Brown | Violet to purple | Green | Unknown |
Dangers
The toxicity of 5-MeO-MiPT is not known. There is no known documentation of death attributed to the use of 5-MeO-MiPT alone.
Legal status
Canada
5-MeO-MiPT is not scheduled in Canada .[citation needed]
China
As of October 2015 5-MeO-MiPT is a controlled substance in China.[11]
Finland
Scheduled in government decree on psychoactive substances banned from the consumer market.[12]
Luxembourg
In Luxembourg, 5-MeO-MiPT is not cited in the list of prohibited substances.[13] Therefore, it is still a legal substance.
United Kingdom
5-MeO-MiPT is a Class A drug in the United Kingdom as are most ethers of ring-hydroxy tryptamines.[citation needed]
United States
5-MeO-MiPT is unscheduled at the federal level in the United States ,[14] but it could be considered an analog of 5-MeO-DiPT, in which case purchase, sale, or possession with intent to consume could be prosecuted under the Federal Analog Act.
Florida
"5-Methoxy-N-methyl-N-isopropyltryptamine" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in the state of Florida.[15]
See also
- MIPT
- 5-MeO-DMT
- 5-MeO-DiPT
- 5-MeO-MET
References
- ↑ "Analytical Profiles for Five "Designer" Tryptamines" (PDF). Microgram Journal 3 (1–2): 55. 2004. http://www.erowid.org/library/periodicals/microgram/microgram_journal_2005-1.pdf#page=54. Retrieved 2013-10-09.
- ↑ "DEA Proposes Adding Five Psychedelic Compounds to Schedule 1" (in en-US). 2022-01-25. https://www.lucid.news/dea-proposes-five-psychedelic-compounds-schedule-1/.
- ↑ "A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines". Human Psychopharmacology 35 (1): e2719. January 2020. doi:10.1002/hup.2719. PMID 31909513.
- ↑ "5-MeO-MiPT" (in en-US). https://thedrugclassroom.com/video/5-meo-mipt/.
- ↑ "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens". European Neuropsychopharmacology 26 (8): 1327–1337. August 2016. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf.
- ↑ "#40 5-MEO-MIPT". Erowid Online Books : "TIHKAL". https://www.erowid.org/library/books_online/tihkal/tihkal40.shtml.
- ↑ "5-MeO-MIPT (also 5-Methoxy-N,N-Methylisopropyltryptamine". Erowid Exp: Main Index. https://www.erowid.org/experiences/subs/exp_5MeOMIPT.shtml.
- ↑ "Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents". Journal of Medicinal Chemistry 28 (7): 892–896. July 1985. doi:10.1021/jm00145a007. PMID 4009612.
- ↑ "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–137. March 2007. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ "Psychedelics and the human receptorome". PLOS ONE 5 (2): e9019. February 2010. doi:10.1371/journal.pone.0009019. PMID 20126400. Bibcode: 2010PLoSO...5.9019R.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 1130/2014". https://www.finlex.fi/fi/laki/alkup/2014/20141130. Retrieved 11 July 2023.
- ↑ "Loi du 19 février 1973 concernant la vente de substances médicamenteuses et la lutte contre la toxicomanie" (in fr). Journal officiel du Grand-Duché de Luxembourg. http://legilux.public.lu/eli/etat/leg/loi/1973/02/19/n1/jo.
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.". http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
- ↑ "Chapter 893 - Drug Abuse Prevention and Control". Florida Statutes. http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html.
External links
 |


