Chemistry:DiFMDA
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
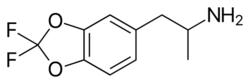
| Formula | C10H11F2NO2 |
| Molar mass | 215.200 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Difluoromethylenedioxyamphetamine (DiFMDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by Daniel Trachsel and coworkers, along with the corresponding fluorinated derivatives of MDMA, MDEA, BDB and MBDB, with the aim of finding a non-neurotoxic drug able to be used as a less harmful substitute for entactogenic drugs such as MDMA. Since a major route of the normal metabolism of these compounds is scission of the methylenedioxy ring, producing neurotoxic metabolites such as alpha-methyldopamine, it was hoped that the difluoromethylenedioxy bioisostere would show increased metabolic stability and less toxicity.[1][2]
These compounds have not yet been tested in animals to verify whether they show similar pharmacological activity to the non-fluorinated parent compounds, although in vitro binding studies show DiFMDA to have a SERT affinity in between that of MDA and MDMA.[3] It is also now generally accepted that MDMA neurotoxicity results from a variety of different causes and is not solely due to accumulation of alpha-methyldopamine,[4][5][6] making it unclear how much less neurotoxic DiFMDA and related drugs would be in practice.
References
- ↑ "Synthesis of fluoro analogues of 3,4-(methylenedioxy)amphetamine (MDA) and its derivatives". Chemistry & Biodiversity 3 (3): 326–36. March 2006. doi:10.1002/cbdv.200690035. PMID 17193269.
- ↑ Meanwell NA (March 2011). "Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design". Journal of Medicinal Chemistry 54 (8): 2529–91. doi:10.1021/jm1013693. PMID 21413808.
- ↑ "Comparative molecular field analysis using selectivity fields reveals residues in the third transmembrane helix of the serotonin transporter associated with substrate and antagonist recognition". The Journal of Pharmacology and Experimental Therapeutics 325 (3): 791–800. June 2008. doi:10.1124/jpet.108.136200. PMID 18354055.
- ↑ "Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview". Molecular Neurobiology 39 (3): 210–71. June 2009. doi:10.1007/s12035-009-8064-1. PMID 19373443.
- ↑ "Neurotoxicity of ecstasy (MDMA): an overview". Current Pharmaceutical Biotechnology 11 (5): 460–9. August 2010. doi:10.2174/138920110791591490. PMID 20420572.
- ↑ "Comparative neurochemical profile of 3,4-methylenedioxymethamphetamine and its metabolite alpha-methyldopamine on key targets of MDMA neurotoxicity". Neurochemistry International 58 (1): 92–101. January 2011. doi:10.1016/j.neuint.2010.11.001. PMID 21074589.

