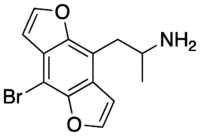
Chemistry:Bromo-DragonFLY
| |
| Names | |
|---|---|
| IUPAC name
1-(4-Bromofuro[2,3-f] [1]benzofuran-8-yl)propan-2-amine[1]
| |
| Other names
Bromo-benzodifuranyl-isopropylamine[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H12BrNO2 | |
| Molar mass | 294.144 g mol−1 |
| log P | 2.519 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromo-DragonFLY (or 3C-Bromo-Dragonfly, DOB-Dragonfly[2]) is a substance related to the phenethylamine family. It acts as a potent full agonist for the 5-HT2A receptor.[3]
History
Bromo-DragonFLY was first synthesized by Matthew Parker in the laboratory of David E. Nichols in 1998. As with the earlier and less potent dihydrofuran series of compounds nicknamed FLY, Bromo-DragonFLY was named after its superficial structural resemblance to a dragonfly.
Pharmacology
Bromo-DragonFLY demonstrates potent affinity at the 5-HT2A receptor (Ki 0.04 nM), similarly potent affinity at the 5-HT2C receptor (Ki 0.02 nM), and moderate affinity for the 5-HT2B receptor (Ki 0.19 nM).[3][4] Bromo-DragonFLY is also a MAO-A inhibitor,[5] and thus strongly inhibits oxidative deamination of 5-HT, increasing its risk profile.
Chemistry
The first synthesis of racemic Bromo-DragonFLY was reported by David E. Nichols in 1998 and was an expansion upon earlier research into the tetrahydrobenzodifuran analogue of DOB.[6] The 1998 synthesis of racemic Bromo-DragonFLY starts from hydroquinone, which is dialkylated with 1-bromo-2-chloroethane, brominated, and treated with n-butyllithium to yield the tetrahydrobenzodifuran ring system. After formylation of the ring system, the nitropropene derivative was obtained by condensation with nitroethane under ammonium acetate catalysis. The nitropropene derivative was then reduced with lithium aluminium hydride to yield the amine intermediate, which was protected with trifluoroacetic anhydride. Following para-bromination with elemental bromine and oxidation of the tetrahydrobenzodifuran ring system with DDQ, the trifluoroacetyl protecting group of the amine was removed to give Bromo-DragonFLY as a racemic mixture of the R and S enantiomers.
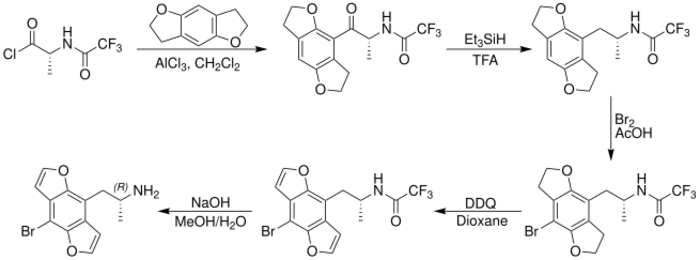
In 2001, David E. Nichols reported an enantiospecific synthesis of Bromo-DragonFLY which allowed the individual R and S enantiomers to be studied.[7] Further research determined that (R)-(-)-Bromo-DragonFLY possessed greater binding affinity at the 5-HT2A and 5-HT2C receptors than (S)-(-)-Bromo-DragonFLY. To synthesize the more active R enantiomer, a derivative of D-alanine was reacted with 2,3,6,7-tetrahydrobenzodifuran in a Friedel–Crafts acylation, yielding an intermediate containing a β-keto moiety which was removed by treatment with triethylsilane in trifluoroacetic acid. After para-bromination and oxidation of the ring system with DDQ, the amine was deprotected yielding (R)-(-)-Bromo-DragonFLY.
Dosage
Data on toxicological significance and dosage of Bromo-DragonFLY remains elusive due to lack of human consumption, however commonly reported recreational dose of this substance is in the range of 500-1000μg.[8] However, a death has been reported at approximately 700μg Bromo-DragonFLY.[8]
Toxicity

The toxicity of Bromo-DragonFLY appears to be fairly high for humans, with reports of at least five deaths believed to have resulted from Bromo-DragonFLY in Norway ,[9] Sweden,[10][11] Denmark,[12][13] Finland[14] and the United States. Laboratory testing has confirmed that in October 2009, a batch of Bromo-Dragonfly was distributed, mislabeled as the related compound 2C-B-FLY, which is around 20x less potent than BDF by weight. This mistake is believed to have contributed to several lethal overdoses and additional hospitalizations. The batch implicated in these deaths also contained significant synthesis impurities, which may have contributed to the toxicity.[15]
Vasoconstrictive action resulting from severe overdose of Bromo-DragonFLY is known to have caused tissue necrosis of the extremities in at least one case. In September 2007, a 35-year-old Swedish male required amputation of the front part of his feet and several fingers on one hand after taking a massive (but unknown) overdose; reportedly, the compound acted as a long-acting efficacious vasoconstrictor, leading to necrosis and gangrene which became apparent several weeks after the overdose occurred. Treatment was of limited efficacy in this case, although tolazoline is reportedly an effective treatment where available.[16][17]
Overdose-associated disturbing experiences and health problems have been described. One case in 2008 in England involved inhalation of vomit, causing nearly fatal asphyxia.[18] Seizures have also been reported.[19]
On October 3, 2009, a 22-year-old male from Copenhagen died after ingesting Bromo-DragonFLY. His friend described the trip saying, "It was like being dragged to hell and back again. Many times. It is the most evil [thing] I've ever tried. It lasted an eternity."[20]
On May 7, 2011, in the United States, two young adults died after overdosing on Bromo-DragonFLY, which they thought was 2C-E, and several others were hospitalized during the same incident. Because they took a dosage appropriate for 2C-E, those who took the drug received, in some cases, 100x the normal dose. Both deaths followed seizures, vomiting blood, and terrifying hallucinations.[21]
Drug prohibition laws
United States
Bromo-DragonFLY is unscheduled at federal level in the United States but could be prosecuted if it is sold for human consumption, Bromo Dragonfly is listed as a Schedule I in Oklahoma.[citation needed]
Canada
As of Oct 12, 2016, Bromo-DragonFLY is listed in Schedule III of the Canadian Controlled Drugs and Substances Act: "2C-phenethylamines and their salts, derivatives, isomers and salts of derivatives and isomers", a broad definition which corresponds to anything with a 2,5-dimethoxyphenethylamine core, including (but not limited to) the 2C family (including e.g. βk-2C-B), the DOx chemical class, the TMA family, Aleph aka DOT, NBOMe, the 25x-NBx series, and of course, Bromo-DragonFLY itself (see this article).
United Kingdom
Bromo-DragonFLY is widely reported by the media as being a Class A drug.[22] However, as of 2014, it remains unclear to what extent it is covered by the UK phenylethylamine catch-all clause, with commentators noting both the structural similarities[23] and differences[24][unreliable source?] to the phenylethylamine class. If the prosecution could demonstrate structural similarity in court, it would be considered a Class A substance[25] but as a benzodifuran it is significantly different to this class. It is not explicitly named in the misuse of drugs act.[26] It would be covered by the UK Psychoactive Substances Act 2016 but only if is sold or traded for human consumption.
Sweden
Sveriges riksdag added Bromo-Dragonfly to schedule IV ("substances, plant materials and fungi that hasn't any or without nothing medical use") as narcotics in Sweden as of Jan 3, 2008, published by Medical Products Agency in their regulation LVFS 2007:14 listed as Bromo-Dragonfly, brombensodifuranyl-isopropylamin.[27] Bromo-DragonFLY was first classified as "health hazard" by Sveriges riksdags health ministry Statens folkhälsoinstitut (sv) under the act Lagen om förbud mot vissa hälsofarliga varor (sv) (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Jul 15, 2007, in their regulation SFS 2007:600 listed as brombensodifuranylisopropylamin (Bromo-Dragonfly), making it illegal to sell, purchase, buy, retail or possess.[28]
Denmark
On December 3, 2007, the drug was banned in Denmark.[29] The substance has been declared illegal by health minister Jakob Axel Nielsen, following recommendations from the Danish Health Ministry. It is currently classified as a dangerous narcotic and therefore its possession, manufacture, importation, supply or usage is strictly prohibited. Anyone involved in such activities can face legal action.[30]
Norway
Bromo-DragonFLY is currently on the Norwegian narcotics list.[31][32]
Poland
Currently, Bromo-DragonFLY is an uncontrolled substance in Poland.[citation needed]
Romania
The chemical compound has been added as an illegal substance under the Law 143/2000 on February 10, 2010.[33]
Australia
As of 9 September 2011, Bromo-DragonFLY was added to Schedule 2 of the Queensland Drugs Misuse Regulation 1987.[34]
Nationally, the drug is listed under Schedule 9 (Prohibited) of the Poisons Standard. Accordingly, the drug is prohibited in all states and territories.[35]
Finland
As of 12 March 2012, Bromo-DragonFLY is an illegal designer drug.[36]
See also
Notes
- ↑ "Bromo-DragonFLY". Forendex. USA: Southern Association of Forensic Scientists. http://forendex.southernforensic.org/index.php/detail/index/1125.
- ↑ "Erowid Bromo-Dragonfly Vault". https://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly.shtml.
- ↑ 3.0 3.1 "A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor". Journal of Medicinal Chemistry 41 (26): 5148–9. December 1998. doi:10.1021/jm9803525. PMID 9857084.
- ↑ Corazza, O; Schifano, F; Farre, M; Deluca, P; Davey, Z; Torrens, M; Demetrovics, Z; Di Furia, L et al. (May 2011). "Designer drugs on the internet: a phenomenon out-of-control? the emergence of hallucinogenic drug Bromo-Dragonfly". Current Clinical Pharmacology 6 (2): 125–9. doi:10.2174/157488411796151129. PMID 21592070.
- ↑ "Bromo-dragonfly, a psychoactive benzodifuran, is resistant to hepatic metabolism and potently inhibits monoamine oxidase A". Toxicology Letters 295: 397–407. 1 October 2018. doi:10.1016/j.toxlet.2018.07.018. PMID 30036687.
- ↑ 6.0 6.1 Parker, Matthew; Marona-Lewicka, Danuta; Lucaites, Virginia; Nelson, David; Nichols, David (8 June 1998). "A Novel (Benzodifuranyl)aminoalkane with Extremely Potent Activity at the 5-HT2A Receptor". Journal of Medicinal Chemistry 41 (26): 5148–5149. doi:10.1021/jm9803525. PMID 9857084.
- ↑ 7.0 7.1 Chambers, James; Kurrasch-Orbaugh, Deborah; Parker, Matthew; Nichols, David (26 January 2001). "Enantiospecific Synthesis and Pharmacological Evaluation of a Series of Super-Potent, Conformationally Restricted 5-HT2A/2C Receptor Agonists". Journal of Medicinal Chemistry 44 (14): 1003–1010. doi:10.1021/jm060272y. PMID 16821786.
- ↑ 8.0 8.1 "A fatal poisoning involving Bromo-Dragonfly". Forensic Science International 183 (1): 91–96. 10 January 2009. doi:10.1016/j.forsciint.2008.11.001. PMID 19091499. https://www.sciencedirect.com/science/article/abs/pii/S0379073808004295.
- ↑ "Erowid Bromo-Dragonfly Vault : Reported GHB-Related Death Involved Bromo-Dragonfly". http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_death2.shtml.
- ↑ Kajsa Hallberg (2007-04-03). "Man i 20-årsåldern dog av drogen Dragonfly" (in sv). expressen.se (AB Kvällstidningen Expressen). http://www.expressen.se/Nyheter/1.622826.
- ↑ Ritzau (2008-08-24). "Nyt stof har slået dansker ihjel" (in da). jp.dk. http://jp.dk/indland/aar/article1418250.ece.
- ↑ Andreasen MF; Telving R; Birkler RI; Schumacher B; Johannsen M (January 2009). "A fatal poisoning involving Bromo-Dragonfly". Forensic Science International 183 (1–3): 91–6. doi:10.1016/j.forsciint.2008.11.001. PMID 19091499.
- ↑ Siukonen, Timo (2012-12-14). "Kassisurman päätekijälle 11,5 vuoden vankeustuomio taposta" (in fi). Helsingin Sanomat. http://www.hs.fi/kotimaa/Kassisurman+p%C3%A4%C3%A4tekij%C3%A4lle+115+vuoden+vankeustuomio+taposta/a1305551347404.
- ↑ "Erowid 2C-B-Fly Vault: Death Report". http://www.erowid.org/chemicals/2cb_fly/2cb_fly_death1.shtml.
- ↑ Bromo-dragonfly – livsfarlig missbruksdrog (Swedish)
- ↑ Thorlacius, K; Borna, C; Personne, M (2008). "[Bromo-dragon fly--life-threatening drug. Can cause tissue necrosis as demonstrated by the first described case]". Läkartidningen 105 (16): 1199–200. PMID 18522262.
- ↑ George, Sallie (2008-03-27). "England | Surrey | 'I nearly died from taking £5 hit'". BBC News. http://news.bbc.co.uk/1/hi/england/surrey/7315020.stm.
- ↑ "Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY". Journal of Medical Toxicology 5 (4): 226–9. December 2009. doi:10.1007/bf03178273. PMID 19876858.
- ↑ "| Danish man died after trip on Chinese drug". Jp.dk. 13 November 2009. http://jp.dk/indland/kbh/article1884968.ece.
- ↑ "Second Victim Dies After Taking Designer Drug In Konawa". http://www.newson6.com/story/14641463/second-victim-dies-after-taking-designer-drug-in-konawa.
- ↑ George, Sallie (2008-03-27). "I nearly died from taking £5 hit". BBC News (BBC). http://news.bbc.co.uk/1/hi/england/surrey/7315020.stm.
- ↑ Psychonaut Webmapping Research Group. "Bromo-Dragonfly Report". Institute of Psychiatry, London. http://www.psychonautproject.eu/documents/reports/Bromodragonfly.pdf.
- ↑ "Ask Erowid : ID 3103 : Does Bromo-Dragonfly fall under the UK ban on phenethylamines?". http://www.erowid.org/ask/ask.php?ID=3103.
- ↑ Advisory Council on the Misuse of Drugs. "Consideration of the Novel Psychoactive Substances ('Legal Highs')". UK Home Office. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/119139/acmdnps2011.pdf.
- ↑ UK Home Office. "UK Misuse of Drugs Act 1971 > 1971 c. 38 > SCHEDULE 2". http://www.legislation.gov.uk/ukpga/1971/38/schedule/2.
- ↑ "Läkemedelsverkets föreskrifter - LVFS och HSLF-FS | Läkemedelsverket". http://www.lakemedelsverket.se/upload/lvfs/LVFS_2007-14.pdf.
- ↑ "Archived copy". http://www.notisum.se/rnp/sls/sfs/20070600.pdf.
- ↑ "Amendment of Executive Order on Euphoriant Substances". Danish Medicines Agency. 2007. http://www.dkma.dk/da?sc_itemid=%7BF2733320-09EF-4477-AA70-0725C65FD9FD%7D&path=/1024/visUKLSArtikel.asp?artikelID=12407.
- ↑ "Erowid Bromo-Dragonfly Vault : Legal Status". http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_law.shtml#denmark.
- ↑ "List of narcotic drugs according to Norwegian law". http://www.lovdata.no/for/sf/ho/to-19780630-0008-001.html.
- ↑ "Statens Legemiddelverk about derivates and Bromo-DragonFLY". http://www.legemiddelverket.no/templates/InterPage____57471.aspx.
- ↑ "Modified Romanian law 143/2000 on January 10, 2010.". http://www.legestart.ro/Ordonanta-de-urgenta-6-2010-modificarea-completarea-Legii-143-2000-prevenirea-combaterea-traficului-consumului-ilicit-droguri-completarea-Legii-339-2005-regimul-juridic-plantelor-(MzQ3MzAw).htm.
- ↑ "Queensland Drugs Misuse Regulation 1987". http://www.legislation.qld.gov.au/LEGISLTN/CURRENT/D/DrugsMisuseR87.pdf.
- ↑ Poisons Standard October 2015 "Poisons Standard October 2015". https://www.comlaw.gov.au/Details/F2015L01534.
- ↑ Design drugs (in Finnish)
References
- Parker, MA; Marona-Lewicka, D; Lucaites, VL; Nelson, DL; Nichols, DE (December 1998). "A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor". J. Med. Chem. 41 (26): 5148–5149. doi:10.1021/jm9803525. PMID 9857084. http://www.erowid.org/references/refs_view.php?A=ShowDoc1&ID=6517.
- Chambers, James J. (2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT2A/2C receptor agonists". Journal of Medicinal Chemistry 44 (6): 1003–1010. doi:10.1021/jm000491y. PMID 11300881.
- Bromo-dragonfly – livsfarlig missbruksdrog
- Per, 35, blev stympad av dödsdrogen - Vårdades i respirator i tio dygn efter att ha tagit Bromo-Dragonfly
- [1]
- [2]
 |