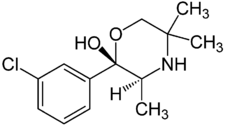
Chemistry:Radafaxine
 | |
| Clinical data | |
|---|---|
| Other names | (S,S)-Hydroxybupropion; (2S,3S)-Hydroxybupropion; GW-353,162 |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H18ClNO2 |
| Molar mass | 255.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Radafaxine (developmental code name GW-353,162), also known as (2S,3S)-hydroxybupropion or (S,S)-hydroxybupropion,[1] is a norepinephrine–dopamine reuptake inhibitor (NDRI) which was under development by GlaxoSmithKline in the 2000s for a variety of different indications but was never marketed.[2] These uses included treatment of restless legs syndrome, major depressive disorder, bipolar disorder, neuropathic pain, fibromyalgia, and obesity.[2] Regulatory filing was planned for 2007,[3] but development was discontinued in 2006 due to "poor test results".[4]
Pharmacology
Pharmacodynamics
Radafaxine is described as a norepinephrine–dopamine reuptake inhibitor (NDRI). In contrast to bupropion, it appears to have a higher potency on inhibition of norepinephrine reuptake than on dopamine reuptake. Radafaxine has about 70% of the efficacy of bupropion in blocking dopamine reuptake, and 392% of efficacy in blocking norepinephrine reuptake, making it fairly selective for inhibiting the reuptake of norepinephrine over dopamine.[5][6] This, according to GlaxoSmithKline, may account for the increased effect of radafaxine on pain and fatigue.[7] At least one study suggests that radafaxine has a low abuse potential similar to bupropion.[8]
Chemistry
Radafaxine is a potent metabolite of bupropion, the compound in GlaxoSmithKline's Wellbutrin. More specifically, "hydroxybupropion" is an analogue of bupropion, and radafaxine is an isolated isomer ((2S,3S)-) of hydroxybupropion.[9] Therefore, radafaxine builds on at least some of the properties of bupropion in humans.[3] Another analogue of bupropion, manifaxine (GW-320,659), was derived from radafxine and was also studied.[10]
See also
References
- ↑ "Bupropion and Bupropion Analogs as Treatments for CNS Disorders". Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Advances in Pharmacology. 69. 2014. pp. 177–216. doi:10.1016/B978-0-12-420118-7.00005-6. ISBN 9780124201187.
- ↑ 2.0 2.1 "Radafaxine - AdisInsight". https://adisinsight.springer.com/drugs/800017221.
- ↑ 3.0 3.1 "Reviews Novel Therapeutics For CNS Disorders And Confirms Strong Pipeline Momentum". BioSpace. 23 November 2004. http://www.biospace.com/news_story.aspx?StoryID=18222420&full=1.
- ↑ Kollewe, Julia (27 July 2006). "GSK breakthrough on bird flu vaccine". Independent.co.uk. http://news.independent.co.uk/business/news/article1199374.ece#10919204097707524565.
- ↑ "Stereoselective analysis of hydroxybupropion and application to drug interaction studies". Chirality 19 (3): 163–70. March 2007. doi:10.1002/chir.20356. PMID 17167747.
- ↑ "Behavioral and biochemical investigations of bupropion metabolites". European Journal of Pharmacology 474 (1): 85–93. August 2003. doi:10.1016/S0014-2999(03)02010-7. PMID 12909199.
- ↑ Burch, Daniel. "Neurosciences Development Portfolio". http://213.219.8.102/pdfs/gsk/cns_seminar/353162.pdf.
- ↑ "The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects". Biological Psychiatry 57 (6): 640–6. March 2005. doi:10.1016/j.biopsych.2004.12.007. PMID 15780851.
- ↑ Radafaxine at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ "Manifaxine - AdisInsight". https://adisinsight.springer.com/drugs/800006906.
External links
 |

