Chemistry:Mecamylamine
 | |
| Clinical data | |
|---|---|
| Trade names | Inversine, Vecamyl |
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 40% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
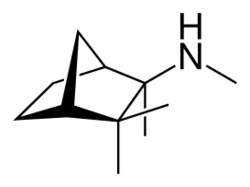
| Formula | C11H21N |
| Molar mass | 167.296 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mecamylamine (INN, BAN; or mecamylamine hydrochloride (USAN); brand names Inversine, Vecamyl[1]) is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug.[2] In the United States , it was voluntarily withdrawn from the market in 2009 but was brought to market in 2013 as Vecamyl and eventually was marketed by Turing Pharmaceuticals.[3][4]
Chemically, mecamylamine is a secondary aliphatic amine, with a pKaH of 11.2[5]
Medical uses
Mecamylamine has been used as an orally-active ganglionic blocker in treating autonomic dysreflexia and hypertension,[6] but, like most ganglionic blockers, it is more often used now as a research tool.
Mecamylamine is also sometimes used as an antiaddictive drug to help people stop smoking tobacco,[7] and is now more widely used for this application than it is for lowering blood pressure. This effect is thought to be due to its blocking α3β4 nicotinic receptors in the brain. It has also been reported to bring about sustained relief from tics in Tourette syndrome when a series of more usually used agents had failed.
In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an augmentation therapy. This is the first substantive evidence that shows that compounds where the primary pharmacology is antagonism to neuronal nicotinic receptors will have antidepressant properties.[8][9] TC-5214 is currently in Phase III of clinical development as an add-on treatment and on stage II as a monotherapy treatment for major depression. The first results reported from the Phase III trials showed that TC-5214 failed to meet the primary goal and the trial did not replicate the effects that had been encouraging in the Phase II trial.[10][11] Development is funded by Targacept and AstraZeneca.[12] It did not produce meaningful, beneficial results on patients as measured by changes on the Montgomery-Asberg Depression Rating Scale after eight weeks of treatment as compared with placebo.
In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an augmentation therapy. This is the first substantive evidence that shows that compounds where the primary pharmacology is antagonism to neuronal nicotinic receptors will have antidepressant properties.[8][9] TC-5214 is currently in Phase III of clinical development as an add-on treatment and on stage II as a monotherapy treatment for major depression. The first results reported from the Phase III trials showed that TC-5214 failed to meet the primary goal and the trial did not replicate the effects that had been encouraging in the Phase II trial.[13][14] Development is funded by Targacept and AstraZeneca.[15] It did not produce meaningful, beneficial results on patients as measured by changes on the Montgomery-Asberg Depression Rating Scale after eight weeks of treatment as compared with placebo. In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an augmentation therapy. This is the first substantive evidence that shows that compounds where the primary pharmacology is antagonism to neuronal nicotinic receptors will have antidepressant properties.[8][9] TC-5214 is currently in Phase III of clinical development as an add-on treatment and on stage II as a monotherapy treatment for major depression. The first results reported from the Phase III trials showed that TC-5214 failed to meet the primary goal and the trial did not replicate the effects that had been encouraging in the Phase II trial.[16][17] Development is funded by Targacept and AstraZeneca.[18] It did not produce meaningful, beneficial results on patients as measured by changes on the Montgomery-Asberg Depression Rating Scale after eight weeks of treatment as compared with placebo.
Overdose
The -1">50 for the HCl salt[19] in mice: 21 mg/kg (i.v.); 37 mg/kg (i.p.); 96 mg/kg (p.o.).[20]
Pharmacology
(S)-(+)-Mecamylamine dissociates more slowly from α4β2 and α3β4 receptors than does the (R)-(−)-enantiomer.[21]
A large SAR study of mecamylamine and its analogs was reported by a group from Merck in 1962.[22] Another, more recent SAR study was undertaken by Suchocki et al.[23]
A comprehensive review of the pharmacology of mecamylamine was published in 2001.[24]
History
Mecamylamine was brought to market by Merck & Co. in the 1950s; in 1996 Merck sold the asset to Layton Bioscience.[25] In 2002, Targacept acquired it from Layton, intending to repurpose it for CNS conditions.[26] Targacept voluntarily withdrew mecamylamine from the market in 2009[27] for reasons not related to safety or efficacy.[28] Manchester Pharmaceuticals brought the drug back to market in 2013.[29] Retrophin acquired Manchester in 2014[30] and after Martin Shkreli was forced out of Retrophin, in 2014 his new company, Turing Pharmaceuticals, acquired the rights to mecamylamine from Retrophin.[31]
See also
- Bupropion
- Scopolamine
References
- ↑ "Mecamylamine". drugs.com. https://www.drugs.com/mtm/mecamylamine.html.
- ↑ "Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders". Expert Opinion on Pharmacotherapy 10 (16): 2709–2721. November 2009. doi:10.1517/14656560903329102. PMID 19874251.
- ↑ "Drug Profile: Mecamylamine - Targacept". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800014114.
- ↑ "Drugs@FDA: FDA Approved Drug Products". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=204054.
- ↑ "Absorption of drugs from the stomach. I. The rat". The Journal of Pharmacology and Experimental Therapeutics 120 (4): 528–539. August 1957. PMID 13476377.
- ↑ Textbook of Organic Medicinal and Pharmaceutical Chemistry (5th ed.). Philadelphia: Lippincott. 1966. pp. 468–546.
- ↑ "Mecamylamine (Inversine): an old antihypertensive with new research directions". Journal of Human Hypertension 16 (7): 453–457. July 2002. doi:10.1038/sj.jhh.1001416. PMID 12080428.
- ↑ 8.0 8.1 8.2 "TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity". CNS Neuroscience & Therapeutics 14 (4): 266–277. 2008. doi:10.1111/j.1755-5949.2008.00054.x. PMID 19040552.
- ↑ 9.0 9.1 9.2 "The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice". Psychopharmacology 189 (3): 395–401. December 2006. doi:10.1007/s00213-006-0568-z. PMID 17016705.
- ↑ "Key AZ/Targacept depression drug flunks first Phase III test". Fiercebiotech.com. 8 November 2011. http://www.fiercebiotech.com/story/key-aztargacept-depression-drug-flunks-first-phase-iii-test/2011-11-08.
- ↑ "AstraZeneca, Targacept drug fails depression test". Reuters. 8 November 2011. https://www.reuters.com/article/us-astrazeneca-targacept-idINTRE7A71KO20111108.
- ↑ "AstraZeneca Pipeline as of the 27th of January 2011". http://www.astrazeneca.com/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobheadername1=Content-Disposition&blobheadername2=MDT-Type&blobheadervalue1=inline%3B+filename%3DDownload-pipeline-summary.pdf&blobheadervalue2=abinary%3B+charset%3DUTF-8&blobkey=id&blobtable=MungoBlobs&blobwhere=1285619440126&ssbinary=true.
- ↑ "Key AZ/Targacept depression drug flunks first Phase III test". Fiercebiotech.com. 8 November 2011. http://www.fiercebiotech.com/story/key-aztargacept-depression-drug-flunks-first-phase-iii-test/2011-11-08.
- ↑ "AstraZeneca, Targacept drug fails depression test". Reuters. 8 November 2011. https://www.reuters.com/article/us-astrazeneca-targacept-idINTRE7A71KO20111108.
- ↑ "AstraZeneca Pipeline as of the 27th of January 2011". http://www.astrazeneca.com/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobheadername1=Content-Disposition&blobheadername2=MDT-Type&blobheadervalue1=inline%3B+filename%3DDownload-pipeline-summary.pdf&blobheadervalue2=abinary%3B+charset%3DUTF-8&blobkey=id&blobtable=MungoBlobs&blobwhere=1285619440126&ssbinary=true.
- ↑ "Key AZ/Targacept depression drug flunks first Phase III test". Fiercebiotech.com. 8 November 2011. http://www.fiercebiotech.com/story/key-aztargacept-depression-drug-flunks-first-phase-iii-test/2011-11-08.
- ↑ "AstraZeneca, Targacept drug fails depression test". Reuters. 8 November 2011. https://www.reuters.com/article/us-astrazeneca-targacept-idINTRE7A71KO20111108.
- ↑ "AstraZeneca Pipeline as of the 27th of January 2011". http://www.astrazeneca.com/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobheadername1=Content-Disposition&blobheadername2=MDT-Type&blobheadervalue1=inline%3B+filename%3DDownload-pipeline-summary.pdf&blobheadervalue2=abinary%3B+charset%3DUTF-8&blobkey=id&blobtable=MungoBlobs&blobwhere=1285619440126&ssbinary=true.
- ↑ In view of the time period when these data were generated, they presumably refer to the HCl salt of the racemic drug
- ↑ "The pharmacological actions of pempidine and its ethyl homologue". British Journal of Pharmacology and Chemotherapy 13 (4): 501–520. December 1958. doi:10.1111/j.1476-5381.1958.tb00246.x. PMID 13618559.
- ↑ "Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics 297 (2): 646–656. May 2001. PMID 11303054. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11303054.
- ↑ "Chemistry and Structure-Activity Relationships of Mecamylamine and Derivatives". Journal of Medicinal and Pharmaceutical Chemistry 91 (4): 665–690. July 1962. doi:10.1021/jm01239a001. PMID 14061006.
- ↑ "Synthesis of 2-exo- and 2-endo-mecamylamine analogues. Structure-activity relationships for nicotinic antagonism in the central nervous system". Journal of Medicinal Chemistry 34 (3): 1003–1010. March 1991. doi:10.1021/jm00107a019. PMID 2002445.
- ↑ "Mecamylamine: new therapeutic uses and toxicity/risk profile". Clinical Therapeutics 23 (4): 532–565. April 2001. doi:10.1016/s0149-2918(01)80059-x. PMID 11354389.
- ↑ "Mecamylamine (Inversine): an old antihypertensive with new research directions". Journal of Human Hypertension 16 (7): 453–457. July 2002. doi:10.1038/sj.jhh.1001416. PMID 12080428.
- ↑ "Press release: Targacept, Inc. Acquires Marketed Drug To Expand Its CNS Portfolio | Evaluate". Targacept via Evaluate. August 27, 2002. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=86220§ionID=&isEPVantage=no.
- ↑ "Notification letter from Targacept". FDA. June 4, 2009. https://www.fda.gov/downloads/Drugs/NewsEvents/UCM167332.pdf.
- ↑ "Determination That INVERSINE (Mecamylamine Hydrochloride) Tablet and Six Other Drug Products Were Not Withdrawn From Sale for Reasons of Safety or Effectiveness". Federal Register. 28 July 2011. https://www.federalregister.gov/documents/2011/07/28/2011-19110/determination-that-inversine-mecamylamine-hydrochloride-tablet-and-six-other-drug-products-were-not.
- ↑ "Press release: Manchester Announces FDA Approval of Vecamyl". Manchester Pharamceuticals via Evaluate. May 1, 2013. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=487170.
- ↑ "Retrophin Shares Boom Following Manchester Pharma Buyout". Xconomy. 13 February 2014. http://www.xconomy.com/new-york/2014/02/13/retrophin-shares-boom-following-manchester-pharma-buyout/.
- ↑ "Shkreli Leads $90M Round for New Startup, Turing Pharma | Xconomy". Xconomy. 10 August 2015. http://www.xconomy.com/new-york/2015/08/10/shkreli-leads-90m-round-for-new-startup-turing-pharma/#.
 |

