Chemistry:Citicoline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Neurocoline |
| Other names | Cytidine diphosphate choline |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90% oral |
| Excretion | respiration (as CO2) and urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H27N4O11P2+ |
| Molar mass | 489.335 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Citicoline (INN), also known as cytidine diphosphate-choline (CDP-Choline) or cytidine 5'-diphosphocholine is an intermediate in the generation of phosphatidylcholine from choline, a common biochemical process in cell membranes. Citicoline is naturally occurring in the cells of human and animal tissue, in particular the organs.
Use as a dietary supplement
Citicoline is available as a supplement in over 70 countries under a variety of brand names: CereBleu, Cebroton, Ceraxon, Cidilin, Citifar, Cognizin, Difosfocin, Hipercol, NeurAxon, Nicholin, Sinkron, Somazina, Synapsine, Startonyl, Trausan, Xerenoos, etc.[1] When taken as a supplement, citicoline is hydrolyzed into choline and cytidine in the intestine.[2] Once these cross the blood–brain barrier it is reformed into citicoline by the rate-limiting enzyme in phosphatidylcholine synthesis, CTP-phosphocholine cytidylyltransferase.[3][4]
Research
Memory and cognition
Studies suggest, but have not confirmed, potential benefits of citicoline for cognitive impairments.[5]
Ischemic stroke
Some preliminary research suggested that citicoline may reduce the rates of death and disability following an ischemic stroke.[6][7] However, the largest citicoline clinical trial to date (a randomised, placebo-controlled, sequential trial of 2,298 patients with moderate-to-severe acute ischaemic stroke in Europe), found no benefit of administering citicoline on survival or recovery from stroke.[8] A meta-analysis of seven trials reported no statistically significant benefit for long-term survival or recovery.[9]
Vision
The effect of citicoline on visual function has been studied in patients with glaucoma, with possible positive effect for protecting vision.[10]
Mechanism of action
Neuroprotective effects
Citicoline may have neuroprotective effects due to its preservation of cardiolipin and sphingomyelin, preservation of arachidonic acid content of phosphatidylcholine and phosphatidylethanolamine, partial restoration of phosphatidylcholine levels, and stimulation of glutathione synthesis and glutathione reductase activity. Citicoline's effects may also be explained by the reduction of phospholipase A2 activity.[11] Citicoline increases phosphatidylcholine synthesis.[12][13][14] The mechanism for this may be:
- By converting 1, 2-diacylglycerol into phosphatidylcholine
- Stimulating the synthesis of SAMe, which aids in membrane stabilization and reduces levels of arachidonic acid. This is especially important after an ischemia when arachidonic acid levels are elevated.[15]
Neuronal membrane
The brain preferentially uses choline to synthesize acetylcholine. This limits the amount of choline available to synthesize phosphatidylcholine. When the availability of choline is low or the need for acetylcholine increases, phospholipids containing choline can be catabolized from neuronal membranes. These phospholipids include sphingomyelin and phosphatidylcholine.[11] Supplementation with citicoline can increase the amount of choline available for acetylcholine synthesis and aid in rebuilding membrane phospholipid stores after depletion.[16] Citicoline decreases phospholipase stimulation. This can lower levels of hydroxyl radicals produced after an ischemia and prevent cardiolipin from being catabolized by phospholipase A2.[17][18] It can also work to restore cardiolipin levels in the inner mitochondrial membrane.[17]
Cell signalling
Citicoline may enhance cellular communication by increasing levels of neurotransmitters.[19] The choline component of citicoline is used to create acetylcholine, which is a neurotransmitter in the human brain. Clinical trials have found that citicoline supplementation might improve focus and attention.[20]
Glutamate transport
Citicoline lowers increased glutamate concentrations and raises decreased ATP concentrations induced by ischemia. Citicoline also increases glutamate uptake by increasing expression of EAAT2, a glutamate transporter, in vitro in rat astrocytes. It is suggested that the neuroprotective effects of citicoline after a stroke are due in part to citicoline's ability to decrease levels of glutamate in the brain.[21]
Pharmacokinetics
Citicoline is water-soluble, with more than 90% oral bioavailability.[16] Plasma levels of citicholine peak one hour after oral ingestion, and a majority of the citicoline is excreted as CO
2 in respiration with the remaining citicoline being excreted through urine.[22] The pharmacokinetic profile of citicholine cannot be described by a single smooth exponential decrease over time.[22] However, the elimination half-life for citicholine has been reported as approximately 50 hours for citicholine removed via respiration and approximately 70 hours for citicholine removed via urine.[22] Plasma levels of choline peak about four hours after ingestion.[23]
Side effects
Citicoline has a very low toxicity profile in animals and humans. Clinically, doses of 2000 mg per day have been observed and approved. Minor transient adverse effects are rare and most commonly include stomach pain and diarrhea.[13][unreliable medical source?] There have been suggestions that chronic citicoline use may have adverse psychiatric effects. However, a meta-analysis of the relevant literature does not support this hypothesis.[24][25] At most, citicoline may exacerbate psychotic episodes or interact with antipsychotic medication.
Synthesis
In vivo
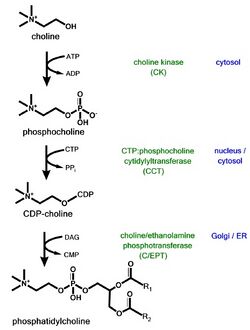
Phosphatidylcholine is a major phospholipid in eukaryotic cell membranes. Close regulation of its biosynthesis, degradation, and distribution is essential to proper cell function. Phosphatidylcholine is synthesized in vivo by two pathways
- The Kennedy pathway, which includes the transformation of choline to citicoline, by way of phosphorylcholine, produces phosphatidylcholine when condensed with diacylglycerol.
- Phosphatidylcholine can also be produced by the methylation pathway, where phosphatidylethanolamine is sequentially methylated.[26]
See also
- 1-alkenyl-2-acylglycerol choline phosphotransferase
- Ceramide cholinephosphotransferase
- Choline-phosphate cytidylyltransferase
- Diacylglycerol cholinephosphotransferase
- Sphingosine cholinephosphotransferase
References
- ↑ Single-ingredient Preparations (: Citicoline). In: Martindale: The Complete Drug Reference [ed.by Sweetman S], 35th Ed. 2007, The Pharmaceutical Press: London (UK); e-version. .
- ↑ "Effect of oral CDP-choline on plasma choline and uridine levels in humans". Biochemical Pharmacology 60 (7): 989–92. Oct 2000. doi:10.1016/S0006-2952(00)00436-6. PMID 10974208.
- ↑ "Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats". Methods and Findings in Experimental and Clinical Pharmacology 21 (8): 535–40. Oct 1999. doi:10.1358/mf.1999.21.8.794835. PMID 10599052.
- ↑ "Antidepressant-like effects of cytidine in the forced swim test in rats". Biological Psychiatry 51 (11): 882–9. Jun 2002. doi:10.1016/s0006-3223(01)01344-0. PMID 12022961.
- ↑ "The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives". Clin Interv Aging 10: 1421–9. 2015. doi:10.2147/CIA.S87886. PMID 26366063.
- ↑ "Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators". Annals of Neurology 48 (5): 713–22. Nov 2000. doi:10.1002/1531-8249(200011)48:5<713::aid-ana4>3.0.co;2-#. PMID 11079534.
- ↑ "Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair". Reviews in Neurological Diseases 5 (4): 167–77. Fall 2008. PMID 19122569.
- ↑ "Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial)". Lancet 380 (9839): 349–57. Jul 2012. doi:10.1016/S0140-6736(12)60813-7. PMID 22691567.
- ↑ "Early application of citicoline in the treatment of acute stroke: A meta-analysis of randomized controlled trials". J. Huazhong Univ. Sci. Technol. Med. Sci. 36 (2): 270–7. 2016. doi:10.1007/s11596-016-1579-6. PMID 27072975.
- ↑ "Cytidine 5'-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives". Int J Mol Sci 16 (12): 28401–17. 2015. doi:10.3390/ijms161226099. PMID 26633368.
- ↑ 11.0 11.1 "Citicoline: neuroprotective mechanisms in cerebral ischemia". Journal of Neurochemistry 80 (1): 12–23. Jan 2002. doi:10.1046/j.0022-3042.2001.00697.x. PMID 11796739.
- ↑ "Evidence that 5'-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels". Journal of Neurochemistry 65 (2): 889–94. Aug 1995. doi:10.1046/j.1471-4159.1995.65020889.x. PMID 7616250.
- ↑ 13.0 13.1 "Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature". Alternative Medicine Review 9 (1): 17–31. Mar 2004. PMID 15005642.
- ↑ "Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study". Psychopharmacology 161 (3): 248–54. May 2002. doi:10.1007/s00213-002-1045-y. PMID 12021827.
- ↑ "CDP-choline: neuroprotection in transient forebrain ischemia of gerbils". Journal of Neuroscience Research 58 (5): 697–705. Dec 1999. doi:10.1002/(sici)1097-4547(19991201)58:5<697::aid-jnr11>3.0.co;2-b. PMID 10561698.
- ↑ 16.0 16.1 "Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury". Neurological Research 17 (4): 281–4. Aug 1995. doi:10.1080/01616412.1995.11740327. PMID 7477743.
- ↑ 17.0 17.1 "Does CDP-choline modulate phospholipase activities after transient forebrain ischemia?". Brain Research 893 (1–2): 268–72. Mar 2001. doi:10.1016/S0006-8993(00)03280-7. PMID 11223016.
- ↑ "Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia". Journal of Neuroscience Research 73 (3): 308–15. Aug 2003. doi:10.1002/jnr.10672. PMID 12868064.
- ↑ "Citicoline: pharmacological and clinical review, 2006 update". Methods and Findings in Experimental and Clinical Pharmacology 28 (Suppl B): 1–56. Sep 2006. PMID 17171187.
- ↑ "The use of citicoline for the treatment of cognitive decline and cognitive impairment: A meta-analysis of pharmacological literature • International Journal of Environmental Science & Technology" (in en-US). 2020-08-30. https://www.ijest.org/citicoline-cognitive-decline-ptardner-0820/.
- ↑ "Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport". Neurobiology of Disease 18 (2): 336–345. Mar 2005. doi:10.1016/j.nbd.2004.10.006. PMID 15686962.
- ↑ 22.0 22.1 22.2 "Pharmacokinetics of 14C CDP-choline". Arzneimittel-Forschung 33 (7A): 1066–1070. 1983. PMID 6412727.
- ↑ "Metabolism of cytidine (5?)-diphosphocholine (cdp-choline) following oral and intravenous administration to the human and the rat". Neurochemistry International 11 (3): 293–297. January 1987. doi:10.1016/0197-0186(87)90049-0. PMID 20501174.
- ↑ "Can Citicoline Cause Depression?: A review of the clinical literature • International Journal of Environmental Science & Technology" (in en-US). 2020-08-28. https://www.ijest.org/citicoline-depression-ptardner-0820/.
- ↑ "Probable Nootropicinduced Psychiatric Adverse Effects: A Series of Four Cases". Innovations in Clinical Neuroscience 12 (11–12): 21–25. 2015. PMID 27222762.
- ↑ "Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway". The Journal of Biological Chemistry 280 (46): 38290–6. Nov 2005. doi:10.1074/jbc.M507700200. PMID 16172116.
 |