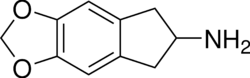
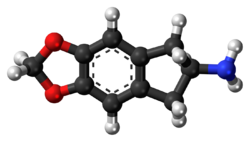
Chemistry:MDAI
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H11NO2 |
| Molar mass | 177.203 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
Chemistry
The chemical structure of MDAI is indirectly derived from that of the illicit drug MDA, but the alpha-methyl group of the alkyl amino amphetamine side chain has been bound back to the benzene nucleus to form an indane ring system, which changes its pharmacological properties substantially.
MDAI can be produced from 3-(3,4-methylenedioxyphenyl)propionic acid[1] which is converted to the acid chloride and then heated to produce 5,6-Methylenedioxy-1-indanone. Treatment of the indanone with amyl nitrite in methanol with HCl afforded the hydroxyimino ketone. This is reduced to the 2-aminoindan following a modification of Nichols' earlier method from a paper discussing DOM analogues,[2] using a Pd/C catalyst in glacial acetic acid with catalytic H2SO4.
Pharmacology
MDAI has been shown to inhibit the reuptake of serotonin, dopamine, and norepinephrine with IC50 values of 512 nM, 5,920 nM, and 1,426 nM, respectively. This demonstrates that MDAI has selective affinity for the serotonin transporter (SERT). In animals treated with reserpine and MDAI, greater extracellular concentrations of monoamine neural transmitters resulted, most significantly serotonin. This result indicates that MDAI is a potent releaser of serotonin, while effectively inhibiting the reuptake of serotonin. For comparison, MDAI is similar in potency with releasing serotonin to MDA but significantly less potent than MDMA.[3]
Effects
The family of drugs typified by MDMA produce their effects through multiple mechanisms of action in the body, and consequently produce three distinct cues which animals can be trained to respond to: a stimulant cue typified by drugs such as methamphetamine, a psychedelic cue typified by drugs such as LSD and DOM, and an "entactogen-like" cue which is produced by drugs such as MDAI and MBDB. These drugs cause drug-appropriate responses in animals trained to recognize the effects of MDMA, but do not produce responses in animals trained selectively to respond to stimulants or hallucinogens. Because these compounds selectively release serotonin in the brain but have little effect on dopamine or noradrenaline levels, they can produce empathogenic effects but without any stimulant action, instead being somewhat sedating.[4][5][6][7][8][9][10]
Very high doses can be fatal in rats with a 50% fatality rate for those subcutaneously injected with 28 mg/kg of MDAI. This is a result of the way serotonin release interferes with thermoregulation.[11]
Use in scientific research
MDAI and other similar drugs have been widely used in scientific research, as they are able to replicate many of the effects of MDMA, but without causing the neurotoxicity which may be associated with MDMA and some related drugs. No tests have been performed on cardiovascular toxicity.[1][12][13][14][15][16][17]
Use as a recreational drug
MDAI has been advertised as a designer drug. It started to be sold online from around 2007, but reached peak popularity between about 2010–2012, after bans on mephedrone came into effect in various countries. Internet-sourced products claimed to be MDAI have been shown variously to contain mephedrone or other substituted cathinone derivatives, and mixed compositions of inorganic substances, while generally containing no MDAI. The number of internet searches for MDAI has been considerably higher in the UK compared to Germany and the USA.[18] MDAI is only non-neurotoxic in isolation but may become neurotoxic when mixed with other drugs.[19] Three deaths were linked to MDAI use in the UK during 2011–2012, all involving symptoms consistent with serotonin syndrome. Two of these also involved other drugs while one death appeared to be from MDAI alone.[20]
Legal Status
As of October 2015 MDAI is a controlled substance in China.[21]
MDAI is illegal in Denmark as of September 2015.[22]
As of December 2011 MDAI is a controlled substance in Switzerland [23]
See also
References
- ↑ 1.0 1.1 "Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA)". Journal of Medicinal Chemistry 33 (2): 703–10. February 1990. doi:10.1021/jm00164a037. PMID 1967651.
- ↑ "Potential psychotomimetics. 2. Rigid analogs of 2,5-dimethoxy-4-methylphenylisopropylamine (DOM, STP)". Journal of Medicinal Chemistry 17 (2): 161–6. February 1974. doi:10.1021/jm00248a004. PMID 4809251.
- ↑ "[3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues". European Journal of Pharmacology 200 (1): 9–16. July 1991. doi:10.1016/0014-2999(91)90659-e. PMID 1685125.
- ↑ "Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain". Biochemical Pharmacology 36 (14): 2297–303. July 1987. doi:10.1016/0006-2952(87)90594-6. PMID 2886126.
- ↑ "Drug discrimination studies with MDMA and amphetamine". Psychopharmacology 95 (1): 71–6. 1988. doi:10.1007/bf00212770. PMID 2898791.
- ↑ "Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens". Journal of Psychoactive Drugs 18 (4): 305–13. 1986. doi:10.1080/02791072.1986.10472362. PMID 2880944.
- ↑ "(+)-N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity". The Journal of Pharmacology and Experimental Therapeutics 255 (3): 1098–106. December 1990. PMID 1979813.
- ↑ "Structural variation and (+)-amphetamine-like discriminative stimulus properties". Pharmacology, Biochemistry, and Behavior 38 (3): 581–6. March 1991. doi:10.1016/0091-3057(91)90017-V. PMID 2068194. PMID
- ↑ "Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan". European Journal of Pharmacology 258 (1–2): 1–13. June 1994. doi:10.1016/0014-2999(94)90051-5. PMID 7925587.
- ↑ "Chemistry and pharmacology of hallucinogens, entactogens and stimulants". Pharmacopsychiatry 31 (Suppl 2): 69–72. July 1998. doi:10.1055/s-2007-979349. PMID 9754836.
- ↑ "Emerging toxicity of 5,6-methylenedioxy-2-aminoindane (MDAI): Pharmacokinetics, behaviour, thermoregulation and LD50 in rats". Progress in Neuro-Psychopharmacology & Biological Psychiatry 69: 49–59. August 2016. doi:10.1016/j.pnpbp.2016.04.004. PMID 27083855.
- ↑ "5-Iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine". Pharmacology, Biochemistry, and Behavior 38 (1): 135–9. January 1991. doi:10.1016/0091-3057(91)90601-w. PMID 1826785.
- ↑ "Synthesis and pharmacological examination of 1-(3-methoxy-4-methylphenyl)-2-aminopropane and 5-methoxy-6-methyl-2-aminoindan: similarities to 3,4-(methylenedioxy)methamphetamine (MDMA)". Journal of Medicinal Chemistry 34 (5): 1662–8. May 1991. doi:10.1021/jm00109a020. PMID 1674539.
- ↑ "Serotonin neurotoxicity in rats after combined treatment with a dopaminergic agent followed by a nonneurotoxic 3,4-methylenedioxymethamphetamine (MDMA) analogue". Pharmacology, Biochemistry, and Behavior 40 (4): 915–22. December 1991. doi:10.1016/0091-3057(91)90106-c. PMID 1726189.
- ↑ "Novel serotonergic agents". Drug Design and Discovery 9 (3–4): 299–312. 1993. PMID 8400010.
- ↑ "Studies on the mechanism of p-chloroamphetamine neurotoxicity". Biochemical Pharmacology 52 (8): 1271–7. October 1996. doi:10.1016/0006-2952(96)00482-0. PMID 8937435.
- ↑ "Indan analogs of fenfluramine and norfenfluramine have reduced neurotoxic potential". Pharmacology, Biochemistry, and Behavior 59 (3): 709–15. March 1998. doi:10.1016/s0091-3057(97)00557-1. PMID 9512076.
- ↑ "5,6-Methylenedioxy-2-aminoindane: from laboratory curiosity to 'legal high'". Human Psychopharmacology 27 (2): 106–12. March 2012. doi:10.1002/hup.1255. PMID 22389075.
- ↑ "Second generation mephedrone. The confusing case of NRG-1". BMJ 341: c3564. July 2010. doi:10.1136/bmj.c3564. PMID 20605894.
- ↑ "MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; 'sparkle'; 'mindy') toxicity: a brief overview and update". Human Psychopharmacology 28 (4): 345–55. July 2013. doi:10.1002/hup.2298. PMID 23881883.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Lists of euphoriant substances subject to control in Denmark". The Danish Medicines Agency. September 2015. http://laegemiddelstyrelsen.dk/en/licensing/company-authorisations-and-registrations/euphoriant-substances/lists.
- ↑ "812.121.11" (in de). Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien (Regulation of the EDI about the directories of drugs, psychotropic substances, precursors and auxiliary chemicals). Das Eidgenössische Departement des Innern (EDI). December 2011. https://www.admin.ch/opc/de/classified-compilation/20101220/201112010000/812.121.11.pdf.
 |


