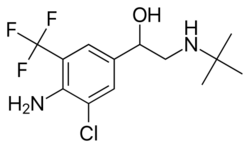
Chemistry:Mabuterol
 | |
| Clinical data | |
|---|---|
| Other names | Mabuterolum; PB 868Cl |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H18ClF3N2O |
| Molar mass | 310.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mabuterol is a selective β2 adrenoreceptor agonist.[1][2]
Synthesis
The halogenation of 2-(Trifluoromethyl)aniline [88-17-5] (1) with iodine and sodium bicarbonate resulted in 2-Amino-5-Iodobenzotrifluoride [97760-97-9] (2). Protection with acetic anhydride followed by nucleophilic aromatic displacement with copper(I)cyanide gave N-[4-cyano-2-(trifluoromethyl)phenyl]acetamide [175277-96-0] (3). Hydrolysis of the nitrile and the protecting group gave 4-amino-3-(trifluoromethyl)benzoic acid [400-76-0] (4). Halogenation with chlorine gave 4-Amino-3-Chloro-5-(Trifluoromethyl)Benzoic Acid [95656-52-3] (5). Halogenation of the acid with thionyl chloride gave 4-Amino-3-chloro-5-(trifluoromethyl)benzoylchloride [63498-15-7] (6). Treatment with diethyl malonate [105-53-3] gave the acetophenone and hence 1-[4-amino-3-chloro-5-(trifluoromethyl)phenyl]ethanone [97760-76-4] (7). Halogenation with bromine in acetic acid led to 1-[4-amino-3-chloro-5-(trifluoromethyl)phenyl]-2-bromoethanone [97760-87-7] (8). Treatment with tert-butylamine [75-64-9] yielded 1-[4-amino-3-chloro-5-(trifluoromethyl)phenyl]-2-(tert-butylamino)ethenone, CID:13355601 (9). Reduction of the ketone with sodium borohydride completed the synthesis of Mabuterol (10).
See also
- Clenbuterol
- Cimaterol
- Trantinterol (regioisomer)
References
- ↑ "Pharmacological studies of mabuterol, a new selective beta 2-stimulant. II: Effects on the cardiovascular system and smooth muscle organs". Arzneimittel-Forschung 34 (11A): 1641–1651. 1984. PMID 6152157.
- ↑ "Beta-adrenoceptor blocking effects of a selective beta 2-agonist, mabuterol, on the isolated, blood-perfused right atrium of the dog". British Journal of Pharmacology 97 (3): 709–716. July 1989. doi:10.1111/j.1476-5381.1989.tb12007.x. PMID 2474351.
- ↑ "Synthesis of further amino-halogen-substituted phenyl-aminoethanols". Arzneimittel-Forschung 34 (11A): 1612–24. 1984. PMID 6152154.
- ↑ Engelhardt G, Keck J, "Aminophenyl ethanolamines and oxazolidines - having analgesic, uterus spasmolytic and anti-spasmodic activity on cross-striped muscle structure", BE patent 808743, issued 1974, assigned to Thomae GmbH.
- ↑ US patent 4119710, issued 1978, assigned to Boehringer Ing.
- ↑ DE patent 2351281, assigned to Thomae GmbH.
- ↑ "Synthesis of stable isotope labeled D9 -Mabuterol, D9 -Bambuterol, and D9 -Cimbuterol". Journal of Labelled Compounds & Radiopharmaceuticals 59 (13): 546–551. November 2016. doi:10.1002/jlcr.3446. PMID 27739098.
 |

