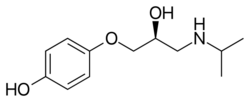
Chemistry:Prenalterol
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H19NO3 |
| Molar mass | 225.288 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Prenalterol is a cardiac stimulant which acts as a β1 adrenoreceptor agonist.[1]
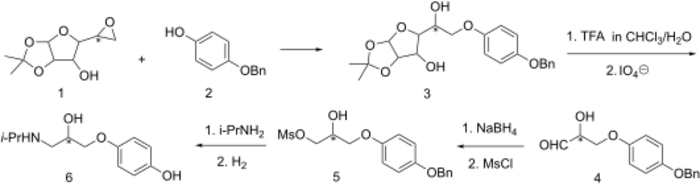
Synthesis
Stereospecific
Prenalterol exhibits adrenergic agonist activity in spite of an interposed oxymethylene group. The stereospecific synthesis devised for this molecule relies on the fact that the side chain is very similar in oxidation state to that of a sugar.
Condensation of monobenzone (2) with the epoxide derived from α-D-glucofuranose[4] affords the glycosylated derivative (3). Hydrolytic removal of the acetonide protecting groups[5] followed by cleavage of the sugar with periodate gives aldehyde (4). This is reduced to the glycol by means of NaBH4 and the terminal alcohol is converted to the mesylate (5). Displacement of the leaving group with isopropylamine followed by hydrogenolytic removal of the O-benzyl ether affords the β1-adrenergic selective adrenergic agonist prenalterol (6).
Racemic
Prepns of the racemic mixture: NL patent 6409883 corresp to H. Köppe et al., U.S. Patent 3,637,852 (1965, 1972 both to Boehringer Ingelheim); NL patent 301580 corresp to A. F. Crowther, L. H. Smith, U.S. Patent 3,501,769 (1965, 1970 both to ICI);[6]
See also
References
- ↑ "The cardiovascular pharmacology of xamoterol, cicloprolol, prenalterol and pindolol in the anaesthetised dog". British Journal of Clinical Pharmacology 28 (Suppl 1): 78S–81S. 1989. doi:10.1111/j.1365-2125.1989.tb03580.x. PMID 2572262.
- ↑ Jaeggi KA, Schröter H, Ostermayer F, "Optisch aktive Derivate des 1-Phenoxy-2-hydroxy-3-aminopropan und Verfahren zu ihrer Herstellung [Optically active derivatives of 1-phenoxy-2-hydroxy-3-aminopropane and the process for their production]", DE patent 2503968, published 1975-08-14 Chem. Abstr. 84, 5322 (1976).
- ↑ corresp to U.S. Patent 3,978,041 and U.S. Patent 4,049,797 (1975, 1976, 1977, all to Ciba-Geigy).
- ↑ "Α-D-Glucofuranose | C6H12O6". ChemSpider. http://www.chemspider.com/Chemical-Structure.9312824.html.
- ↑ "Acetonide Protection of Dopamine for the Synthesis of Highly Pure N-docosahexaenoyldopamine". Tetrahedron Letters 51 (18): 2403–2405. May 2010. doi:10.1016/j.tetlet.2010.02.089. PMID 20543896.
- ↑ "Beta-Adrenergic blocking agents. V. 1-Amino-3-(substituted phenoxy)-2-propanols". Journal of Medicinal Chemistry 12 (4): 638–642. July 1969. doi:10.1021/jm00304a018. PMID 5793156.
Further reading
- "Special Issue on Prenalterol". Acta Medica Scandinavica 211 (S659). 1982. doi:10.1111/joim.1982.211.issue-s659.
 |