Chemistry:Thioproperazine
 | |
| Clinical data | |
|---|---|
| Trade names | Majeptil |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
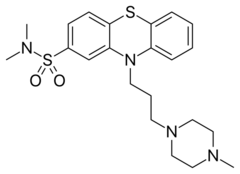
| Formula | C22H30N4O2S2 |
| Molar mass | 446.63 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Thioproperazine, sold under the brand name Majeptil, is a typical antipsychotic of the phenothiazine group which is used as a tranquilizer, antiemetic, sedative, and in the treatment of schizophrenia and manic phase of bipolar disorder.[1][2][3][4] Majeptil is available in 10 mg tablets.[5]
Side effects
Common[6]
- Extrapyramidal symptoms
- Amenorrhea
- Decreased sexual interest and/or function
- Swelling of breasts and milk production in males and females
- Difficulty sleeping
- Constipation
- Reduced amount of urine
- Dizziness
- Drowsiness
- Dry mouth
- Nausea
- Headache
- Weight changes
Rare but potentially serious adverse effects
- Agranulocytosis
- Neuroleptic Malignant Syndrome (NMS)
- Sudden cardiac death
- Torsades de pointes
Elderly individuals with dementia-related psychosis treated with antipsychotic medication are at an increased risk of death compared to individuals not receiving antipsychotics.
Drug interactions
Medications for allergies (e.g., Benadryl diphenhydramine), certain medications for sleep (e.g., lorazepam, zopiclone), certain medications for pain (e.g., fentanyl), and Antiparkinson medications can increase the sedative effect of thioproperazine and can be potentially dangerous when used together.
Synthesis
Thioether formation between 2-Aminothiophenol (1) and 4-Chloro-N,N-Dimethyl-3-Nitrobenzenesulfonamide [137-47-3] (2) gives 4-(2-aminophenyl)sulfanyl-N,N-dimethyl-3-nitrobenzenesulfonamide [5510-56-5] (3). Sandmeyer reaction with cuprous bromide [7787-70-4] gave 4-[(2-Bromophenyl)-thio]-N,N'-dimethyl-3-nitro-benzenesulfonamide [5510-58-7] (4). Bechamp reduction gave 3-Amino-4-((2-bromophenyl)thio)-N,N-dimethylbenzenesulphonamide [5592-64-3] (5). Goldberg reaction completed the formation of the phenothiazine ring and gave N,N-dimethyl-10H-phenothiazine-2-sulfonamide [1090-78-4] (6). Attachment of the sidechain by sodamide reaction with 1-(3-Chloropropyl)-4-Methylpiperazine [104-16-5] (7) completes the synthesis of Thioproperazine (8), respectively.
References
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1019–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA1019.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 272–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA272.
- ↑ "Phenothiazines (Systemic)". Drugs.com. https://www.drugs.com/international/thioproperazine.html.
- ↑ "Thioproperazine - Oral" (in en-US). https://myhealth.alberta.ca:443/Health/medications/Pages/conditions.aspx?hwid=fdb4224.
- ↑ "Thioproperazine". https://www.drugbank.ca/drugs/DB01622.
- ↑ "Schizophrenia Society of Ontario - Majeptil (thioproperazine)". http://www.schizophrenia.on.ca/Schizophrenia-Psychosis/Medication-Resource-Centre/Typical-Antipsychotic-Medications/Majeptil-(thioproperazine).
- ↑ "Synthesis and comparison of activity of 2- and 3-dimethylsulfamidophenothiazine derivatives". Pharmaceutical Chemistry Journal 8 (5): 286–290. 1974. doi:10.1007/BF00771334.
- ↑ , GB patent 814512 (1959 to Rhone Poulenc SA).
- ↑ , GB patent 813025 (1959-05-06 to Rhone Poulenc Sa).
- ↑ Robert Michel Jacob, Gilbert Louis Regnier, DE patent 1088964 (1960 to Rhone Poulenc Sa).
 |


