Chemistry:Levosulpiride
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
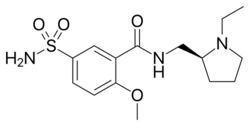
| Formula | C15H23N3O4S |
| Molar mass | 341.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Levosulpiride, sold under the brand name SULPEPTA , is a potent prokinetic agent of the benzamide class.[1] It is a selective antagonist of the dopamine D2 receptors and 5HT4 Agonism [2] on both central and peripheral nervous systems. Levosulpiride is claimed to have mood elevating properties.
Chemically, it is the (S)-(−)-enantiomer of sulpiride.
Uses
Levosulpiride is used in the treatment of:
- Psychosis
- Negative symptoms of schizophrenia
- Anxiety disorders
- Dysthymia
- Vertigo
- Dyspepsia
- Irritable bowel syndrome
- Premature ejaculation.
Levosulpiride is not currently licensed for treatment of premature ejaculation in the UK or other European countries.[3]
Side effects
Side effects include amenorrhea, gynecomastia, galactorrhea, changes in libido, and neuroleptic malignant syndrome.[4] In the U.S., as of 2013 only one case of adverse reaction to levosulpiride had been recorded on the FDA Adverse Event Reporting System Database.[3] A case of rapid onset resistant dystonia caused by low dose levosulpiride was reported in India.[5]
Mechanism of action
In contrast to most other neuroleptics which block both D1 and D2 receptors, levosulpiride is more selective and acts primarily as a D2 antagonist. Levosulpiride appears to lack effects on norepinephrine, acetylcholine, serotonin, histamine, and gamma-aminobutyric acid (GABA) receptors.[6]
Pharmacodynamics
Levosulpiride is a substituted benzamide derivative and a selective dopamine D2 antagonist with antipsychotic and antidepressant activity. Other benzamide derivatives include metoclopramide, tiapride, and sultopride.[6]
See also
References
- ↑ "Levosulpiride - S-(-)-Sulpiride". Generon. http://www.generon.co.uk/amine-oxides-1830/levosulpiride-s-sulpiride-98--231014951.html.
- ↑ "Levosulpiride". Stratech Scientific Ltd. http://www.stratech.co.uk/products/S2104-SEL___levosulpiride.
- ↑ 3.0 3.1 "Antipsychotics and torsadogenic risk: signals emerging from the US FDA Adverse Event Reporting System database". Drug Safety 36 (6): 467–79. June 2013. doi:10.1007/s40264-013-0032-z. PMID 23553446.
- ↑ "Levosulpiride drug information". DrugsUpdate India. http://www.drugsupdate.com/generic/view/860.
- ↑ "Rapid onset resistant dystonia with low dose of Levosulpiride.". British Journal of Psychiatry 190 (1): 81. January 2007. doi:10.1192/bjp.190.1.81a.
- ↑ 6.0 6.1 "Sulpiride". DrugBank. http://www.drugbank.ca/drugs/DB00391.
 |

