Chemistry:RTI-31
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H20ClNO2 |
| Molar mass | 293.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
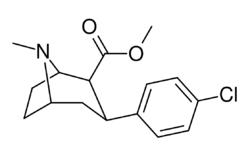
(–)-2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane (RTI-4229-31) is a synthetic analog of cocaine that acts as a stimulant.[1] Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article,[1] RTI-31 is 64 times the strength of cocaine in terms of its potency to elicit self-administration in monkeys. WIN 35428 was 6 times weaker than RTI-31, whereas RTI-51 was 2.6 times weaker than RTI-31.
A further advantage, in addition to potency of this compound, is that its duration of activity is longer than for cocaine. It could therefore be considered within the context as an agonist based therapy for treating cocaine addiction, although it is actually RTI-336 that entered into clinical trials with this in mind. RTI-31 is already completely psychoactive in its own right meaning that further chemical manipulation should be viewed as an option that is not strictly necessary. RTI-336 is actually made using RTI-31 as starting material. RTI-31 is not an entirely selective DRI in that it also has appreciable SERT and NET blocking affinity. RTI-31 can easily be "cleaned" though, as is done, for instance, by replacing the carbomethoxy ester with a more sterically occluded substituent such as is done for RTI-113.
Binding and uptake selectivity
Based on the uptake of tritiated biogenic monoamine radiotracers it can be confirmed by observing the figures in the attached table that RTI-31 is a relatively balanced reuptake inhibitor wrt the D/N/S ratio.
The binding ligand affinities for the different transporters is skewed somewhat in favor of the DAT; there may be some bias in the data. The reason for this could be that WIN35428 is relatively easier to displace from the DAT versus paroxetine from the SERT, because of the higher binding constant of the former compound.
Also it needs to be borne in mind the idea of transporter promiscuity.[2] It may be possible that the NE levels are raised, at least in part, through DAT blockade.
RTI-31 lies somewhere in the middle of the table between troparil on one end and RTI-55 on the other. It is not as selective as RTI-113 for the DAT, but is more selective than Dichloropane is for this transporter. RTI-31 also has some muscarinic acetylcholine agonist activity.
| MAT IC50 (and Ki) for simple phenyltropanes with 1R,2S,3S stereochemistry.[3] | ||||||
| Compound | [3H]CFT | [3H]DA | [3H]Nisoxetine | [3H]NE | [3H]Paroxetine | [3H]5-HT |
| Cocaine[4] | 89.1 | 275 cf. 241 | 3300 (1990) | 119 cf. 161 | 1050 (45) | 177 cf. 112 |
| Troparil | 23 | 49.8 | 920 (550) | 37.2 | 1960 (178) | 173 |
| WIN 35428 | 13.9 | 23.0 | 835 (503) | 38.6 | 692 (63) | 101 |
| RTI-31 | 1.1 | 3.68 | 37 (22) | 5.86 | 44.5 (4.0) | 5.00 |
| RTI-113[5] | 1.98 | 5.25 | 2,926 | 242 | 2,340 | 391 |
| RTI-51 | 1.7 | ? | 37.4 (23) | ? | 10.6 (0.96) | ? |
| RTI-55 | 1.3 | 1.96 | 36 (22) | 7.51 | 4.21 (0.38) | 1.74 |
| RTI-32 | 1.7 | 7.02 | 60 (36) | 8.42 | 240 (23) | 19.4 |
Data in Above table from rats brains (1995). More recent work has advocated using cloned human transporters.
See also
References
- ↑ 1.0 1.1 "A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants". Neuropsychopharmacology 31 (2): 351–62. February 2006. doi:10.1038/sj.npp.1300795. PMID 15957006.
- ↑ "Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy". Pharmacology & Therapeutics 121 (1): 89–99. January 2009. doi:10.1016/j.pharmthera.2008.10.004. PMID 19022290.
- ↑ "Cocaine and 3 beta-(4'-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues. New high-affinity and selective compounds for the dopamine transporter". Journal of Medicinal Chemistry 38 (2): 379–88. January 1995. doi:10.1021/jm00002a020. PMID 7830281.
- ↑ "Mixed cocaine agonist/antagonist properties of (+)-methyl 4beta-(4-chlorophenyl)-1-methylpiperidine-3alpha-carboxylate, a piperidine-based analog of cocaine". The Journal of Pharmacology and Experimental Therapeutics 305 (1): 143–50. April 2003. doi:10.1124/jpet.102.046318. PMID 12649362.
- ↑ "Pharmacological characterization of nicotine's interaction with cocaine and cocaine analogs". The Journal of Pharmacology and Experimental Therapeutics 289 (3): 1229–36. June 1999. PMID 10336510. http://jpet.aspetjournals.org/content/289/3/1229.long.
 |

