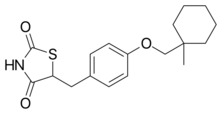
Chemistry:Ciglitazone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H23NO3S |
| Molar mass | 333.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ciglitazone (INN) is a thiazolidinedione. Developed by Takeda Pharmaceuticals in the early 1980s, it is considered the prototypical compound for the thiazolidinedione class.[1][2][3][4]
Ciglitazone was never used as a medication, but it sparked interest in the effects of thiazolidinediones. Several analogues were later developed, some of which—such as pioglitazone and troglitazone—made it to the market.[2]
Ciglitazone significantly decreases VEGF production by human granulosa cells in an in vitro study, and may potentially be used in ovarian hyperstimulation syndrome.[5] Ciglitazone is a potent and selective PPARγ ligand. It binds to the PPARγ ligand-binding domain with an EC50 of 3.0 μM. Ciglitazone is active in vivo as an anti-hyperglycemic agent in the ob/ob murine model.[6] Inhibits HUVEC differentiation and angiogenesis and also stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells.[7]
References
- ↑ "Effects of ciglitazone on blood pressure and intracellular calcium metabolism". Hypertension 21 (6 Pt 2): 1020–1023. June 1993. doi:10.1161/01.hyp.21.6.1020. PMID 8505086.
- ↑ 2.0 2.1 "The glitazone family of antidiabetic agents". Current Pharmaceutical Design 2: 85–102. 1996. doi:10.2174/1381612802666220920215821. https://books.google.com/books?id=IYn4Va7wtAoC&q=ciglitazone&pg=PA86.
- ↑ "Studies on non-thiazolidinedione antidiabetic agents. 1. Discovery of novel oxyiminoacetic acid derivatives". Chemical & Pharmaceutical Bulletin 50 (10): 1349–1357. October 2002. doi:10.1248/cpb.50.1349. PMID 12372861.
- ↑ "[Discovery and development of a new insulin sensitizing agent, pioglitazone]" (in ja). Yakugaku Zasshi 122 (11): 909–918. November 2002. doi:10.1248/yakushi.122.909. PMID 12440149.
- ↑ "Thiazolidinediones decrease vascular endothelial growth factor (VEGF) production by human luteinized granulosa cells in vitro". Fertility and Sterility 93 (6): 2042–2047. April 2010. doi:10.1016/j.fertnstert.2009.02.059. PMID 19342033.
- ↑ "The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones". Journal of Medicinal Chemistry 39 (3): 665–668. February 1996. doi:10.1021/jm950395a. PMID 8576907.
- ↑ "Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo". The Journal of Biological Chemistry 274 (13): 9116–9121. March 1999. doi:10.1074/jbc.274.13.9116. PMID 10085162.
 |

