Chemistry:Rifaximin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Zaxine, Xifaxanta, Normix, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Liver |
| Elimination half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
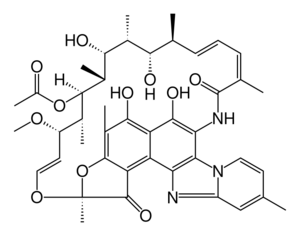
| Formula | C43H51N3O11 |
| Molar mass | 785.891 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 200 to 205 °C (392 to 401 °F) (dec.) |
| |
| |
| | |
Rifaximin, is a non-absorbable, broad spectrum antibiotic mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription.[3] It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.[4]
Medical uses
Travelers' diarrhea
Rifaximin is used to treat travelers' diarrhea (TD) caused by E. coli bacteria in adults and children at least 12 years of age. It treats travelers' diarrhea by stopping the growth of the bacteria that cause diarrhea. Rifaximin will not work to treat travelers' diarrhea that is bloody or occurs with fever.[5]
Irritable bowel syndrome
Rifaximin is used for the treatment of irritable bowel syndrome (IBS). It possesses anti-inflammatory and antibacterial properties, and is a nonabsorbable antibiotic that acts locally in the gut. These properties make it efficacious in relieving chronic functional symptoms of non-constipation type IBS.[6] It appears to retain its therapeutic properties for this indication, even after repeated courses.[7][8] It is particularly indicated where small intestine bacterial overgrowth is suspected of involvement in a person's IBS. Symptom relief or improvement can be obtained for global IBS symptoms, including: abdominal pain, flatulence, bloating, and stool consistency. A drawback is that repeated courses may be necessary for relapse of symptoms.[8][9]
Clostridioides difficile infection
Rifaximin may also be a useful addition to vancomycin when treating patients with relapsing C. difficile infection (CDI).[10][11] However, the quality of evidence of these studies was judged to be low.[12] Because exposure to rifamycins in the past may increase risk for resistance, rifaximin should be avoided in such cases.[13]
Hepatic encephalopathy
Rifaximin is used to prevent episodes of hepatic encephalopathy (changes in thinking, behavior, and personality caused by a build-up of toxins in the brain in people who have liver disease) in adults who have liver disease. It treats hepatic encephalopathy (HE) by stopping the growth of bacteria that produce toxins and that may worsen the liver disease. Although high-quality evidence is still lacking, it appears to be as effective as, or more effective than, other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster.[9][14] It prevents reoccurring encephalopathy and is associated with high patient satisfaction. People are more compliant and satisfied to take this medication than any other due to minimal side effects, prolonged remission, and overall cost.[15] The drawbacks are increased cost, and lack of robust clinical trials for HE without combination lactulose therapy.[16]
Other uses
Other uses include treatment of: infectious diarrhea, small intestinal bacterial overgrowth, inflammatory bowel disease, and diverticular disease.[17][9] It is effective in treating small intestinal bacterial overgrowth regardless of whether it is associated with irritable bowel syndrome or not.[18] It has also shown efficacy with rosacea, ocular rosacea which also presents as dry eyes for patients with co-occurrence with small intestinal bacterial overgrowth (SIBO).[19]
Special caution
Patients should avoid rifaximin if they are allergic to any of rifabutin, rifampin, and rifapentine. It may cause attenuated vaccines (such as typhoid vaccine) not to work well. Health-care professionals should be informed about its usage before receiving immunization.[20] Pregnant or breastfeeding women should avoid rifaximin: it is a pregnancy category C drug and can harm the fetus.[21] Caution is required in persons with cirrhosis who have a Child–Pugh score of C.[9]
Side effects
Rifaximin has an excellent safety profile due to its lack of systemic absorption. Clinical trials did not show any serious adverse events while using the drug. There were no deaths while using it in the clinical trials.[7][8][22]
The most common side effects includes nausea, stomach pain, dizziness, fatigue, headaches, muscle tightening and joint pain. It may also cause reddish discoloration of urine.[23]
The most serious side effects of rifaximin are:
- Clostridioides difficile-associated diarrhea (CDAD)
- Drug-resistant bacterial superinfection
- Severe allergic reactions including hives, rashes and itching
Interactions
As rifaximin is not significantly absorbed from the gut, the great majority of these drug interactions are negligible in people with healthy liver function, so healthcare providers usually do not worry about drug interactions unless liver impairment is present.[7] It may decrease the effectiveness of warfarin, a commonly prescribed anticoagulant, in people with liver problems.[24]
Pharmacology
Rifaximin is a semisynthetic broad spectrum antibacterial drug, derived through chemical modification of the natural antibiotic rifamycin.[25] It has very low bioavailability due to its poor absorption after oral administration. Because of this local action within the gut and the lack of horizontal transfer of resistant genes, the development of bacterial resistance is rare, and most of the drug taken orally stays in the gastrointestinal tract where the infection takes place.[26]
Mechanism of action
Rifaximin interferes with transcription by binding to the β-subunit of bacterial RNA polymerase.[9] This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.[27] This in turn results in a reduction of bacteria populations, including gas-producing bacteria, which may reduce mucosal inflammation, epithelial dysfunction, and visceral hypersensitivity. Rifaximin has broad spectrum antibacterial properties against both gram positive and gram negative anaerobic and aerobic bacteria. As a result of bile acid solubility, its antibacterial action is limited mostly to the small intestine and less so the colon.[9] A resetting of the bacterial composition has also been suggested as a possible mechanism of action for relief of IBS symptoms.[28] Additionally, rifaximin may have a direct anti-inflammatory effect on gut mucosa via modulation of the pregnane X receptor.[28] Other mechanisms for its therapeutic properties include inhibition of bacterial translocation across the epithelial lining of the intestine, inhibition of adherence of bacteria to the epithelial cells, and a reduction in the expression of proinflammatory cytokines.[29]
Availability
In the United States, Salix Pharmaceuticals holds a US Patent for rifaximin and markets the drug under the name Xifaxan.[30] In addition to receiving FDA approval for travelers' diarrhea and (marketing approved for)[31] hepatic encephalopathy, rifaximin received FDA approval for IBS in May 2015.[32] No generic formulation is available in the US and none has appeared due to the fact that the FDA approval process was ongoing. If rifaximin receives full FDA approval for hepatic encephalopathy it is likely that Salix will maintain marketing exclusivity and be protected from generic formulations until March 24, 2017.[31] In 2018, a patent dispute with Teva was settled which delayed a generic in the United States, with the patent set to expire in 2029.[33]
Rifaximin is approved in 33 countries for GI disorders.[34] On August 13, 2013, Health Canada issued a Notice of Compliance to Salix Pharmaceuticals Inc. for the drug product Zaxine.[35] In India, it is available under the brand names Ciboz and Xifapill.[36][37] In Russia and Ukraine the drug is sold under the name Alfa Normix (Альфа Нормикс), produced by Alfa Wassermann S.p.A. (Italy).[38] In 2018, the FDA approved a similar drug by Cosmos Pharmaceuticals called Aemcolo for traveler's diarrhea.[39]
References
- ↑ "Rifaximin international". 2 November 2020. https://www.drugs.com/international/rifaximin.html.
- ↑ "Xifaxan- rifaximin tablet". 1 October 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c5e8e2fd-7087-4b78-9181-cc259c0be2f1.
- ↑ "Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases". Current Opinion in Gastroenterology 26 (1): 17–25. January 2010. doi:10.1097/MOG.0b013e328333dc8d. PMID 19881343.
- ↑ "Rifaximin". https://go.drugbank.com/drugs/DB01220.
- ↑ "Therapy for and prevention of traveler's diarrhea". Clinical Infectious Diseases 45 (Suppl 1): S78–S84. July 2007. doi:10.1086/518155. PMID 17582576.
- ↑ "Rifaximin for the treatment of diarrhea-predominant irritable bowel syndrome". Expert Review of Gastroenterology & Hepatology 10 (4): 431–442. 2016. doi:10.1586/17474124.2016.1140571. PMID 26753693.
- ↑ 7.0 7.1 7.2 "Rifaximin for the treatment of irritable bowel syndrome - a drug safety evaluation". Expert Opinion on Drug Safety 15 (7): 983–991. July 2016. doi:10.1080/14740338.2016.1186639. PMID 27149541.
- ↑ 8.0 8.1 8.2 "Clinical Practice Guidelines for Irritable Bowel Syndrome in Korea, 2017 Revised Edition". Journal of Neurogastroenterology and Motility 24 (2): 197–215. April 2018. doi:10.5056/jnm17145. PMID 29605976.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 "Profile of rifaximin and its potential in the treatment of irritable bowel syndrome". Clinical and Experimental Gastroenterology 8: 159–167. 2015. doi:10.2147/CEG.S67231. PMID 26089696.
- ↑ "Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin". Clinical Infectious Diseases 44 (6): 846–848. March 2007. doi:10.1086/511870. PMID 17304459.
- ↑ "A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection". The Journal of Antimicrobial Chemotherapy 66 (12): 2850–2855. December 2011. doi:10.1093/jac/dkr377. PMID 21948965.
- ↑ "Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults". The Cochrane Database of Systematic Reviews 2017 (3): CD004610. March 2017. doi:10.1002/14651858.CD004610.pub5. PMID 28257555.
- ↑ "Use of rifamycin drugs and development of infection by rifamycin-resistant strains of Clostridium difficile". Antimicrobial Agents and Chemotherapy 57 (6): 2690–2693. June 2013. doi:10.1128/AAC.00548-13. PMID 23545528.
- ↑ "Rifaximin for the treatment of hepatic encephalopathy". Pharmacotherapy 28 (8): 1019–1032. August 2008. doi:10.1592/phco.28.8.1019. PMID 18657018. Free full text with registration at Medscape.
- ↑ "Safety, efficacy, and patient acceptability of rifaximin for hepatic encephalopathy". Patient Preference and Adherence 8: 331–338. March 2014. doi:10.2147/PPA.S41565. PMID 24672227.
- ↑ "Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver". Hepatology 60 (2): 715–735. August 2014. doi:10.1002/hep.27210. PMID 25042402.
- ↑ "Diverticular Disease and Rifaximin: An Evidence-Based Review". Antibiotics 12 (3): 443. February 2023. doi:10.3390/antibiotics12030443. PMID 36978310.
- ↑ "Rifaximin: The Revolutionary Antibiotic Approach for Irritable Bowel Syndrome". Mini Reviews in Medicinal Chemistry 16 (3): 186–192. 2015. doi:10.2174/1389557515666150722105340. PMID 26202193.
- ↑ "Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin". Journal of the American Academy of Dermatology 68 (5): 875–876. May 2013. doi:10.1016/j.jaad.2012.11.038. PMID 23602178.
- ↑ "Rifaximin Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD" (in en). https://www.webmd.com/drugs/2/drug-91339/rifaximin-oral/details.
- ↑ "Fertility and pregnancy in the patient with inflammatory bowel disease". Gut 55 (8): 1198–1206. August 2006. doi:10.1136/gut.2005.078097. PMID 16849349.
- ↑ "Rifaximin", StatPearls (Treasure Island (FL): StatPearls Publishing), 2022, PMID 32966000, http://www.ncbi.nlm.nih.gov/books/NBK562329/, retrieved 2022-06-28
- ↑ "Rifaximin Side Effects". MedlinePlus. U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a604027.html#side-effects.
- ↑ "Probable interaction between warfarin and rifaximin in a patient treated for small intestine bacterial overgrowth". The Annals of Pharmacotherapy 45 (5): e25. May 2011. doi:10.1345/aph.1P578. PMID 21505109.
- ↑ "The synthesis of 4-deoxypyrido[1',2'-1,2]imidazo[5,4-c]rifamycin SV derivatives". The Journal of Antibiotics 37 (12): 1611–1622. December 1984. doi:10.7164/antibiotics.37.1611. PMID 6526730.
- ↑ "Poorly absorbed antibiotics for the treatment of traveler's diarrhea". Clinical Infectious Diseases 41 Suppl 8 (Supplement_8): S564–S570. December 2005. doi:10.1086/432953. PMID 16267720.
- ↑ "Rifaximin". DrugBank. 22 March 2017. http://www.drugbank.ca/drugs/DB01220.
- ↑ 28.0 28.1 "Review article: potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea". Alimentary Pharmacology & Therapeutics 43 (Suppl 1): 37–49. January 2016. doi:10.1111/apt.13437. PMID 26618924.
- ↑ "Pharmacologic Agents for Chronic Diarrhea". Intestinal Research 13 (4): 306–312. October 2015. doi:10.5217/ir.2015.13.4.306. PMID 26576135.
- ↑ "Xifaxan (Rifaximin) 550 mg - Reduce Overt Hepatic Encephalopathy Recurrences". Salix Pharmaceuticals. http://www.salix.com/products/xifaxan550.aspx.
- ↑ 31.0 31.1 "Product Details for NDA 022554". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration (FDA). https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=022554.
- ↑ "FDA approves two therapies to treat IBS-D" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on 2018-01-26. Retrieved 2019-12-16.
- ↑ "Bausch Health stock soars 8.6% premarket on news of patent settlement" (in en-US). https://www.marketwatch.com/story/bausch-health-stock-soars-86-premarket-on-news-of-patent-settlement-2018-09-12.
- ↑ "Pharmaceutical News & Media - Salix Pharmaceuticals". http://www.salix.com/news-media/news/previous-years-news/fda-approves-xifaxan%C2%AE-550-mg-tablets-for-reduction-in-risk-of-overt-hepatic-encephalopathy-he-recurrence.aspx.
- ↑ "Summary Basis of Decision (SBD): Zaxine". Government of Canada, Health Canada, Health Products and Food Branch, Therapeutic Products Directorate, Bureau of Gastroenterology Infection and Viral Diseases. 2013. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2013_zaxine_161256-eng.php.
- ↑ "Brands" (in en-US). https://www.zydushealthcare.com/brands/.
- ↑ "Trade Marks Journal". The Government of India, Trade Mark Registry. 2017-03-20. https://ipindia.gov.in/writereaddata/Portal/IPOJournal/1_451_1/CLASS_1-5.pdf.
- ↑ "Alfa Normix". Russian medical server. http://www.rusmedserv.com/lekarstva/alfa-normiks.html.
- ↑ "Cosmo to give Bausch Health a run for its money with FDA nod for Xifaxan rival" (in en). https://www.fiercepharma.com/marketing/cosmo-to-give-bausch-health-sales-a-run-latest-fda-nod-for-xifaxan-rival.
External links
- "Rifaximin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/rifaximin.
 |

