Chemistry:Furanyl norfentanyl
From HandWiki
Short description: Synthetic opioid analgesic metabolite
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
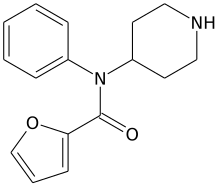
| Formula | C16H18F0N2O2 |
| Molar mass | 270.332 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Furanylnorfentanyl is an inactive synthetic opioid analgesic drug precursor. It is an analog of fentanyl.[1][2][3][4][5]
See also
- 3-Methylbutyrfentanyl
- 4-Fluorobutyrfentanyl
- 4-Fluorofentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Benzylfentanyl
- Furanylfentanyl
- Homofentanyl
- List of fentanyl analogues
References
- ↑ Goggin, Melissa M. (2017). "Identification of unique metabolites of the designer opioid furanyl fentanyl.". Journal of Analytical Toxicology 41 (5): 367–375. doi:10.1093/jat/bkx022. PMID 28369517.
- ↑ Palmquist, Kaitlyn B. (2020). "Quantification of furanylfentanyl and its metabolites in human and rat plasma using LC–MS-MS". Journal of Analytical Toxicology 44 (6): 589–595. doi:10.1093/jat/bkaa013. PMID 32064536.
- ↑ Larabi, Islam Amine (2020). "Hair testing for 3-fluorofentanyl, furanylfentanyl, methoxyacetylfentanyl, carfentanil, acetylfentanyl and fentanyl by LC–MS/MS after unintentional overdose.". Forensic Toxicology 38 (1): 277-286. doi:10.1007/s11419-019-00502-0.
- ↑ Daniulaityte, Raminta (2019). "Street fentanyl use: Experiences, preferences, and concordance between self-reports and urine toxicology.". International Journal of Drug Policy 71: 3–9. doi:10.1016/j.drugpo.2019.05.020. PMID 31146200.
- ↑ Shoemaker, Alyssa K. (2020). Cholinesterase Based System for Fentanyl Detection (MS thesis). State University of New York at Albany.
Further reading
- "Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues". Forensic Toxicology 26 (1): 1–5. June 2008. doi:10.1007/s11419-007-0039-1.
- "Fentanyl receptor assay. II. Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine". J Anal Toxicol 16 (1): 36–41. 1992. doi:10.1093/jat/16.1.36. PMID 1322477.
- "Evaluation of new compounds for opioid activity: 1987 annual report". NIDA Res. Monogr. 81: 543–90. 1988. PMID 3136388.
- "Dependence studies of new compounds in the rhesus monkey, rat, and mouse, 1987". NIDA Res. Monogr. 81: 485–542. 1988. PMID 3136386.
- "Carbon-13 nuclear magnetic resonance spectra of fentanyl analogs". Journal of Heterocyclic Chemistry 26 (3): 677–686. 2009. doi:10.1002/jhet.5570260329.
 |

