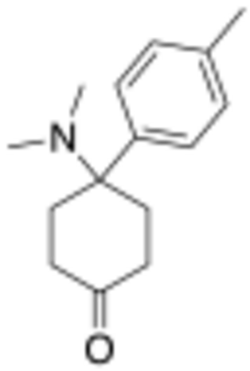
Chemistry:4-Dimethylamino-4-(p-tolyl)cyclohexanone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H21NO |
| Molar mass | 231.339 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
4-Dimethylamino-4-(p-tolyl)cyclohexanone (sometimes known as dimetamine)[1] is a narcotic analgesic with an arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s.[2] It has around the same analgesic potency as morphine, with analogues where the p-methyl group is replaced by chlorine or bromine being slightly weaker. However derivatives where the ketone group has been reacted with a Grignard reagent to add a phenethyl substitution are several hundred times stronger, and in this series it is the bromo compound BDPC that is the most potent.[3][4][5]
Legal Status
4-Dimethylamino-4-(p-tolyl)cyclohexanone is specifically listed as an illegal drug in Latvia.[6] It is also covered by drug analogue laws in various jurisdictions as a generic arylcyclohexylamine derivative.
See also
References
- ↑ This is ambiguous, as Dimetamine is also the name of an unrelated alkaloid, as well as a trade name for propylhexedrine.
- ↑ Lednicer D, "4-Amino-cyclohexanols, their pharmaceutical compositions and methods of use", US patent 4366172, issued 1982-12-28
- ↑ "4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring". Journal of Medicinal Chemistry 23 (4): 424–30. April 1980. doi:10.1021/jm00178a014. PMID 7381841.
- ↑ "4-amino-4-arylcyclohexanones and their derivatives: a novel class of analgesics. 2. Modification of the carbonyl function". Journal of Medicinal Chemistry 24 (4): 404–8. April 1981. doi:10.1021/jm00136a010. PMID 7265128.
- ↑ "4-aryl-4-aminocyclohexanones and their derivatives, a novel class of analgesics. 3. m-Hydroxyphenyl derivates". Journal of Medicinal Chemistry 24 (3): 341–6. March 1981. doi:10.1021/jm00135a019. PMID 7265120.
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem". http://www.vm.gov.lv/images/userfiles/metodiskas_vadlinijas_080914.doc.
 |

