Chemistry:Epiandrosterone
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
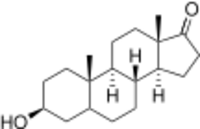
Epiandrosterone, or isoandrosterone,[1][2] also known as 3β-androsterone, 3β-hydroxy-5α-androstan-17-one, or 5α-androstan-3β-ol-17-one, is a steroid hormone with weak androgenic activity. It is a metabolite of testosterone and dihydrotestosterone (DHT). It was first isolated in 1931, by Adolf Friedrich Johann Butenandt and Kurt Tscherning. They distilled over 17,000 litres of male urine, from which they got 50 milligrams of crystalline androsterone (most likely mixed isomers), which was sufficient to find that the chemical formula was very similar to estrone.
Epiandrosterone has been shown to naturally occur in most mammals including pigs.[3]
Epiandrosterone is naturally produced by the enzyme 5α-reductase from the adrenal hormone DHEA.[4][5][6][7][verification needed] Epiandrosterone can also be produced from the natural steroids androstanediol via 17β-hydroxysteroid dehydrogenase or from androstanedione via 3β-hydroxysteroid dehydrogenase.[8]
See also
References
- ↑ Handbook of Aqueous Solubility Data (Second ed.). CRC Press. 19 April 2016. pp. 1209–. ISBN 978-1-4398-0246-5. https://books.google.com/books?id=cfFzJFthLCIC&pg=PA1209.
- ↑ Natural Products. Krishna Prakashan Media. 2006. pp. 298–. ISBN 978-81-87224-85-3. https://books.google.com/books?id=Zb84fVthd_cC&pg=PA298.
- ↑ "Identification of 5 alpha-androstane-3 beta,17 beta-diol and 3 beta-hydroxy-5 alpha-androstan-17-one sulfates as quantitatively significant secretory products of porcine Leydig cells and their presence in testicular venous blood". The Journal of Steroid Biochemistry and Molecular Biology 42 (1): 113–20. March 1992. doi:10.1016/0960-0760(92)90017-d. PMID 1558816.
- ↑ "Influence of oral dehydroepiandrosterone (DHEA) on urinary steroid metabolites in males and females". Steroids 65 (2): 98–102. February 2000. doi:10.1016/s0039-128x(99)00090-2. PMID 10639021.
- ↑ "Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA". The Journal of Steroid Biochemistry and Molecular Biology 110 (1–2): 1–9. May 2008. doi:10.1016/j.jsbmb.2008.02.003. PMID 18359622.
- ↑ van de Kerkhof DH (2001). Steroid profiling in doping analysis (Ph.D. thesis). University Utrecht. hdl:1874/328.
- ↑ "Pharmacokinetics of dehydroepiandrosterone and its metabolites after long-term daily oral administration to healthy young men". Fertility and Sterility 81 (3): 595–604. March 2004. doi:10.1016/j.fertnstert.2003.07.035. PMID 15037408.
- ↑ "Gene structure, chromosomal localization and analysis of 3-ketosteroid reductase activity of the human 3(alpha-->beta)-hydroxysteroid epimerase". Biochimica et Biophysica Acta 1520 (2): 124–30. August 2001. doi:10.1016/s0167-4781(01)00247-0. PMID 11513953.
Further reading
- "Immunoreactive detection of four mammalian steroids in plants.". Canadian Journal of Botany 67 (2): 288–96. February 1989. doi:10.1139/b89-042.
- "Mammalian sex hormones in plants". Folia Histochemica et Cytobiologica 43 (2): 71–9. 2005. PMID 16044944.
- "Bioavailability and metabolism of oral and percutaneous dehydroepiandrosterone in postmenopausal women". The Journal of Steroid Biochemistry and Molecular Biology 107 (1–2): 57–69. October 2007. doi:10.1016/j.jsbmb.2007.02.007. PMID 17627814.
- "Over-the-counter anabolic steroids 4-androsten-3,17-dione; 4-androsten-3beta,17beta-diol; and 19-nor-4-androsten-3,17-dione: excretion studies in men". Journal of Analytical Toxicology 23 (5): 357–66. September 1999. doi:10.1093/jat/23.5.357. PMID 10488924.
 |

