Medicine:Generalized glucocorticoid resistance
| Generalized glucocorticoid resistance | |
|---|---|
| Other names | Chrousos syndrome |
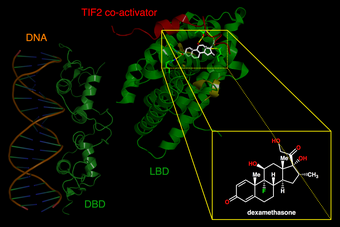 | |
| Left. DNA-binding domains of a glucocorticoid receptor homodimer in the nucleus interacting with DNA. Right. Binding of synthetic glucocorticoid dexamethasone to ligand-binding domain of receptor in cytoplasm. | |
| Specialty | Endocrinology |
Generalized glucocorticoid resistance or Chrousos syndrome is a rare genetic disorder that can run in families or be sporadic. It is characterized by partial or generalized target-tissue insensitivity to glucocorticoids.[1]
The clinical spectrum includes severe, potentially fatal conditions like hypoglycemia, alkalosis, or severe hypokalemia, as well as completely asymptomatic forms. The disease's most prevalent symptom is fatigue.[2]
The elevated 24-hour urinary free cortisol (UFC) excretion in the absence of clinical signs of hypercortisolism and the elevated serum cortisol concentrations point to the diagnosis of generalized glucocorticoid resistance.[3]
The goal of treatment for generalized glucocorticoid resistance is to reduce excessive ACTH secretion, which in turn reduces the production of more adrenal steroids that have androgenic and mineralocorticoid properties.[4] High dosages of synthetic glucocorticoids that spare mineralocorticoids, like dexamethasone, are used as part of the treatment.[5]
Signs and symptoms
Individuals who have generalized glucocorticoid resistance may exhibit biochemical hypercortisolism in the absence of Cushing's syndrome symptoms.[6] The condition's clinical phenotype varies from cases with no symptoms to signs of excess mineralocorticoids in the body such as hypokalemic alkalosis and hypertension and/or androgen excess, including oligospermia in males, menstrual irregularities, hypo fertility, and amenorrhea in females, precocious puberty, male-pattern hair loss, acne, hirsutism, and ambiguous genitalia at birth with 46, XX.[7]
In rare instances, glucocorticoid deficiency has been reported in the following cases: hypoglycemia, severe hypertension, easy "fatigability" with feeding, growth hormone deficiency, and generalized seizures in a 2-year-old girl,[8] adult patients with chronic fatigue,[1][3] and a newborn with hypoglycemia, hypokalemia, and increased arterial pressure.[9]
Causes
De novo genetic defects (point mutations, deletions, or insertions) in the NR3C1 gene can cause sporadic cases of Chrousos syndrome, or it can be inherited in an autosomal recessive or dominant manner.[6][10] Defective human glucocorticoid receptors in the hypothalamus and pituitary cause impaired glucocorticoid negative feedback loops in patients with Chrousos syndrome, which leads to compensatory hypersecretion of adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone (CRH), and arginine vasopressin (AVP).[7]
Mechanism
Steroid hormones known as "glucocorticoids" are produced in the zona fasciculata of the adrenal cortex and numerous other extra-adrenal organs, such as the skin, thymus, and gut.[11] These lipophilic molecules are essential for the maintenance of both resting as well as threatened homeostasis[12] and are secreted in the circulatory system in reaction to stressors[13] and also in an ultradian and circadian manner.[14]
Diagnosis
The elevated 24-hour urinary free cortisol (UFC) excretion with the absence of clinical signs of hypercortisolism and the elevated serum cortisol concentrations point to the diagnosis of generalized glucocorticoid resistance. ACTH plasma concentrations can range from low to high.[3] To confirm the diagnosis, peripheral blood mononuclear cells must be used in thymidine incorporation and dexamethasone-binding assays in conjunction with sequencing of the human glucocorticoid receptor gene.[15]
When diagnosing generalized glucocorticoid resistance, the differential diagnosis consists of additional factors that can lead to hyperandrogenism or virilization, including congenital adrenal hyperplasia, polycystic ovarian syndrome, and idiopathic hirsutism; hyperaldosteronism, essential hypertension, and additional mineralocorticoid-induced hypertensive disorders; circumstances like a typical pregnancy and estrogen therapy that are linked to increased serum concentrations of corticosteroid-binding globulin; pseudo-Cushing's conditions, like melancholic depression and generalized anxiety; and mild variations of Cushing's disease, in which normal or slightly elevated ACTH concentrations coexist with hypercortisolism.[3]
Treatment
The goal of treatment for generalized glucocorticoid resistance is to reduce excessive ACTH secretion, which in turn reduces the production of more adrenal steroids that have androgenic and mineralocorticoid properties.[4] High dosages of mineralocorticoid-sparing synthetic glucocorticoids, like dexamethasone, are used as a form of treatment to activate the mutant and/or wild-type hGRα and suppress the affected subjects' natural secretion of ACTH.[5]
See also
References
- ↑ 1.0 1.1 Chrousos, G P; Vingerhoeds, A; Brandon, D; Eil, C; Pugeat, M; DeVroede, M; Loriaux, D L; Lipsett, M B (June 1, 1982). "Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease.". Journal of Clinical Investigation (American Society for Clinical Investigation) 69 (6): 1261–1269. doi:10.1172/jci110565. ISSN 0021-9738. PMID 6282933.
- ↑ Ruiz, Mini; Lind, Ulrika; Gåfvels, Mats; Eggertsen, Gösta; Carlstedt-Duke, Jan; Nilsson, Lennart; Holtmann, Martin; Stierna, Pontus et al. (2001). "Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance". Clinical Endocrinology 55 (3): 363–371. doi:10.1046/j.1365-2265.2001.01323.x. ISSN 0300-0664. PMID 11589680.
- ↑ 3.0 3.1 3.2 3.3 Charmandari, Evangelia; Kino, Tomoshige; Ichijo, Takamasa; Chrousos, George P. (May 1, 2008). "Generalized Glucocorticoid Resistance: Clinical Aspects, Molecular Mechanisms, and Implications of a Rare Genetic Disorder". The Journal of Clinical Endocrinology & Metabolism (The Endocrine Society) 93 (5): 1563–1572. doi:10.1210/jc.2008-0040. ISSN 0021-972X. PMID 18319312.
- ↑ 4.0 4.1 KINO, TOMOSHIGE; VOTTERO, ALESSANDRA; CHARMANDARI, EVANGELIA; CHROUSOS, GEORGE P. (2002). "Familial/Sporadic Glucocorticoid Resistance Syndrome and Hypertension". Annals of the New York Academy of Sciences (Wiley) 970 (1): 101–111. doi:10.1111/j.1749-6632.2002.tb04416.x. ISSN 0077-8923.
- ↑ 5.0 5.1 Chrousos, George P. (December 1, 1993). "Syndromes of Glucocorticoid Resistance". Annals of Internal Medicine (American College of Physicians) 119 (11): 1113. doi:10.7326/0003-4819-119-11-199312010-00009. ISSN 0003-4819. PMID 8239231.
- ↑ 6.0 6.1 Martins, Clarissa Silva; de Castro, Margaret (2021). "Generalized and tissue specific glucocorticoid resistance". Molecular and Cellular Endocrinology (Elsevier BV) 530: 111277. doi:10.1016/j.mce.2021.111277. ISSN 0303-7207. PMID 33864884.
- ↑ 7.0 7.1 Nicolaides, Nicolas C.; Charmandari, Evangelia (October 7, 2021). "Primary Generalized Glucocorticoid Resistance and Hypersensitivity Syndromes: A 2021 Update". International Journal of Molecular Sciences (MDPI AG) 22 (19): 10839. doi:10.3390/ijms221910839. ISSN 1422-0067. PMID 34639183.
- ↑ Nader, Nancy; Bachrach, Bert E.; Hurt, Darrell E.; Gajula, Sonia; Pittman, Amy; Lescher, Rachel; Kino, Tomoshige (May 1, 2010). "A Novel Point Mutation in Helix 10 of the Human Glucocorticoid Receptor Causes Generalized Glucocorticoid Resistance by Disrupting the Structure of the Ligand-Binding Domain". The Journal of Clinical Endocrinology & Metabolism (The Endocrine Society) 95 (5): 2281–2285. doi:10.1210/jc.2009-2463. ISSN 0021-972X. PMID 20335448.
- ↑ McMahon, Sarah K.; Pretorius, Carel J.; Ungerer, Jacobus P. J.; Salmon, Nathaniel J.; Conwell, Louise S.; Pearen, Michael A.; Batch, Jennifer A. (2010). "Neonatal Complete Generalized Glucocorticoid Resistance and Growth Hormone Deficiency Caused by a Novel Homozygous Mutation in Helix 12 of the Ligand Binding Domain of the Glucocorticoid Receptor Gene (NR3C1)". The Journal of Clinical Endocrinology & Metabolism (The Endocrine Society) 95 (1): 297–302. doi:10.1210/jc.2009-1003. ISSN 0021-972X.
- ↑ Nicolaides, Nicolas C.; Charmandari, Evangelia (2019). "Glucocorticoid Resistance". Experientia Supplementum. Cham: Springer International Publishing. pp. 85–102. doi:10.1007/978-3-030-25905-1_6. ISBN 978-3-030-25904-4.
- ↑ Slominski, Radomir M.; Tuckey, Robert C.; Manna, Pulak R.; Jetten, Anton M.; Postlethwaite, Arnold; Raman, Chander; Slominski, Andrzej T. (March 23, 2020). "Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders". Genes & Immunity (Springer Science and Business Media LLC) 21 (3): 150–168. doi:10.1038/s41435-020-0096-6. ISSN 1466-4879. PMID 32203088.
- ↑ Stavrou, Stavroula; Nicolaides, Nicolas C.; Critselis, Elena; Darviri, Christina; Charmandari, Evangelia; Chrousos, George P. (February 3, 2017). "Paediatric stress: from neuroendocrinology to contemporary disorders". European Journal of Clinical Investigation (Wiley) 47 (3): 262–269. doi:10.1111/eci.12724. ISSN 0014-2972. PMID 28074555.
- ↑ Nicolaides, Nicolas C.; Charmandari, Evangelia; Kino, Tomoshige; Chrousos, George P. (April 28, 2017). "Stress-Related and Circadian Secretion and Target Tissue Actions of Glucocorticoids: Impact on Health". Frontiers in Endocrinology (Frontiers Media SA) 8. doi:10.3389/fendo.2017.00070. ISSN 1664-2392. PMID 28503165.
- ↑ Focke, Caroline M.B.; Iremonger, Karl J. (2020). "Rhythmicity matters: Circadian and ultradian patterns of HPA axis activity". Molecular and Cellular Endocrinology (Elsevier BV) 501: 110652. doi:10.1016/j.mce.2019.110652. ISSN 0303-7207. PMID 31738971.
- ↑ Malchoff, D M; Brufsky, A; Reardon, G; McDermott, P; Javier, E C; Bergh, C H; Rowe, D; Malchoff, C D (May 1, 1993). "A mutation of the glucocorticoid receptor in primary cortisol resistance.". Journal of Clinical Investigation (American Society for Clinical Investigation) 91 (5): 1918–1925. doi:10.1172/jci116410. ISSN 0021-9738. PMID 7683692.
Further reading
- Molnár, Ágnes; Patócs, Attila; Likó, István; Nyírő, Gábor; Rácz, Károly; Tóth, Miklós; Sármán, Beatrix (2018). "An unexpected, mild phenotype of glucocorticoid resistance associated with glucocorticoid receptor gene mutation case report and review of the literature". BMC Medical Genetics 19 (1): 37. doi:10.1186/s12881-018-0552-6. ISSN 1471-2350. PMID 29510671.
- van Rossum, Elisabeth F.C.; Lamberts, Steven W.J. (2006). "Glucocorticoid resistance syndrome: a diagnostic and therapeutic approach". Best Practice & Research Clinical Endocrinology & Metabolism (Elsevier BV) 20 (4): 611–626. doi:10.1016/j.beem.2006.09.005. ISSN 1521-690X. PMID 17161335.
External links
| Classification | |
|---|---|
| External resources |
 |

