Chemistry:Tirbanibulin
 | |
| Clinical data | |
|---|---|
| Trade names | Klisyri |
| Other names | KX2-391 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Topical |
| Drug class | Microtubule inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
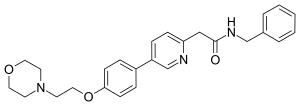
| Formula | C26H29N3O3 |
| Molar mass | 431.536 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tirbanibulin, sold under the brand name Klisyri, is a medication for the treatment of actinic keratosis (AKs) on the face or scalp.[3][4][5] It functions as a mitotic inhibitor by inhibiting tubulin polymerization and Src kinase signaling[6]. It is potentially effective in deferring the development of AKs to squamous cell carcinoma in situ.[7]
The most common side effects include local skin reactions, application site pruritus, and application site pain.[3][4]
Tirbanibulin was approved for medical use in the United States in December 2020,[4][8][9] and in the European Union in July 2021.[5] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[10]
Medical uses
Tirbanibulin is indicated for the topical treatment of actinic keratosis of the face or scalp.[3][4][5]
Mechanism of Action
Tirbanibulin, chemically known as N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl) pyridine-2-yl) acetamide, is a microtubule and non–ATP-competitive inhibitor.[7] The drug in various ways mimics the mechanisms of chemotherapy[11] by suspending the protooncogenic Src tyrosine kinase signaling pathway. Notably, it promotes G2/M arrest during cell cycle, upregulates p53, and triggers apoptosis via caspase-3 stimulation and poly (ADP-ribose) polymerase cleavage.[7]
Side effects
In several studies tirbanibulin has been observed to induce skin reactions at the site of application, ranging from mild to severe erythema, flaking, ulceration, and pain.[11]
As of now, there has been no extensive research conducted on the risks of tirbanibulin usage by specific human populations (i.e., pregnant populations). There also has been no significant differences observed in safety or effectiveness of the drug between geriatric or pediatric populations.[12]
History
The US Food and Drug Administration (FDA) approved tirbanibulin based on evidence from two clinical trials (Trial 1/ NCT03285477 and Trial 2/NCT03285490) of 702 adults with actinic keratosis on the face or scalp.[4] The trials were conducted at 62 sites in the United States.[4] Participants received once daily treatment with either tirbanibulin or inactive control ointment for 5 consecutive days to the single predetermined area where they had actinic keratosis.[4] Neither the participants nor the health care providers knew which treatment was being given until after the trial was completed.[4] The benefit of tirbanibulin in comparison to control was assessed after 57 days by comparing the percentage of participants who did not have any actinic keratosis on the treatment area (100% clearance).[4]
Society and culture
Legal status
On 20 May 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for tirbanibulin, intended for the treatment of actinic keratosis.[13] The applicant for this medicinal product is Almirall, S.A. Tirbanibulin was approved for medical use in the European Union in July 2021.[5][14]
References
- ↑ "Summary Basis of Decision for Onakta". 20 July 2023. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1689944674439.
- ↑ "Details for: Onakta". 12 May 2023. https://dhpp.hpfb-dgpsa.ca/dhpp/resource/102657.
- ↑ 3.0 3.1 3.2 3.3 "Klisyri- tirbanibulin ointment". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=589c8de8-b773-4d47-b60c-48471806cccc.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 "Drug Trials Snapshot: Klisyri". 14 December 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-klisyri.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Klisyri EPAR". 20 April 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/klisyri. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1's low clinical toxicity". The Journal of Biological Chemistry 294 (48): 18099–18108. November 2019. doi:10.1074/jbc.RA119.010732. PMID 31628188.
- ↑ 7.0 7.1 7.2 "1% Tirbanibulin Ointment for the Treatment of Actinic Keratoses". The Annals of Pharmacotherapy 56 (4): 494–500. April 2022. doi:10.1177/10600280211031329. PMID 34301153.
- ↑ "Drug Approval Package: Klisyri". 28 December 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213189Orig1s000TOC.cfm.
- ↑ "Athenex Announces FDA Approval of Klisyri (Tirbanibulin) for the Treatment of Actinic Keratosis on the Face or Scalp" (Press release). Athenex Inc. 15 December 2020. Retrieved 15 December 2020 – via GlobeNewswire.
- ↑ "New Drug Therapy Approvals 2020". 31 December 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2020.
- ↑ 11.0 11.1 "Tirbanibulin (Klisyri) for the Treatment of Actinic Keratosis" (in English). American Family Physician 104 (5): 519–520. November 2021. PMID 34783508. https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=0002838X&v=2.1&it=r&id=GALE%7CA682425390&sid=googleScholar&linkaccess=abs.
- ↑ "Tirbanibulin". American Journal of Health-System Pharmacy 78 (8): 656–657. March 2021. doi:10.1093/ajhp/zxab094. PMID 33787828.
- ↑ "Klisyri: Pending EC decision". 21 May 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/klisyri. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Klisyri Product information". https://ec.europa.eu/health/documents/community-register/html/h1558.htm.
External links
- "Tirbanibulin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tirbanibulin.
- Clinical trial number NCT03285477 for "A Multi-Center Study to Evaluate the Efficacy and Safety of KX2-391 Ointment 1% on AK on Face or Scalp (AK003)" at ClinicalTrials.gov
- Clinical trial number NCT03285490 for "A Multi-Center Study to Evaluate the Efficacy and Safety of KX2-391 Ointment 1% on AK on Face or Scalp (AK004)" at ClinicalTrials.gov
 |

