Chemistry:Sulfate
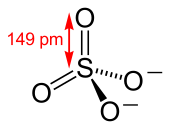
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfate
| |||
| Other names
Tetraoxosulfate(VI)
Tetraoxidosulfate(VI) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| SO2− 4 | |||
| Molar mass | 96.06 g·mol−1 | ||
| Conjugate acid | Hydrogensulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
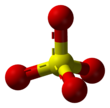
The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−
4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Spelling
"Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English.
Structure
The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, HSO−
4, which is in turn the conjugate base of H
2SO
4, sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of the sulfate ion is as predicted by VSEPR theory.
Bonding
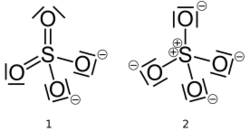
The first description of the bonding in modern terms was by Gilbert Lewis in his groundbreaking paper of 1916 where he described the bonding in terms of electron octets around each atom, that is no double bonds and a formal charge of +2 on the sulfur atom and -1 on each oxygen atom.[1][lower-alpha 1]
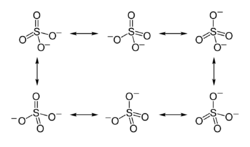
Later, Linus Pauling used valence bond theory to propose that the most significant resonance canonicals had two pi bonds involving d orbitals. His reasoning was that the charge on sulfur was thus reduced, in accordance with his principle of electroneutrality.[2] The S−O bond length of 149 pm is shorter than the bond lengths in sulfuric acid of 157 pm for S−OH. The double bonding was taken by Pauling to account for the shortness of the S−O bond. Pauling's use of d orbitals provoked a debate on the relative importance of pi bonding and bond polarity (electrostatic attraction) in causing the shortening of the S−O bond. The outcome was a broad consensus that d orbitals play a role, but are not as significant as Pauling had believed.[3][4]
A widely accepted description involving pπ – dπ bonding was initially proposed by Durward William John Cruickshank. In this model, fully occupied p orbitals on oxygen overlap with empty sulfur d orbitals (principally the dz2 and dx2–y2).[5] However, in this description, despite there being some π character to the S−O bonds, the bond has significant ionic character. For sulfuric acid, computational analysis (with natural bond orbitals) confirms a clear positive charge on sulfur (theoretically +2.45) and a low 3d occupancy. Therefore, the representation with four single bonds is the optimal Lewis structure rather than the one with two double bonds (thus the Lewis model, not the Pauling model).[6] In this model, the structure obeys the octet rule and the charge distribution is in agreement with the electronegativity of the atoms. The discrepancy between the S−O bond length in the sulfate ion and the S−OH bond length in sulfuric acid is explained by donation of p-orbital electrons from the terminal S=O bonds in sulfuric acid into the antibonding S−OH orbitals, weakening them resulting in the longer bond length of the latter.
However, the bonding representation of Pauling for sulfate and other main group compounds with oxygen is still a common way of representing the bonding in many textbooks.[5][7] The apparent contradiction can be cleared if one realizes that the covalent double bonds in the Lewis structure in reality represent bonds that are strongly polarized by more than 90% towards the oxygen atom. On the other hand, in the structure with a dipolar bond, the charge is localized as a lone pair on the oxygen.[6]
Preparation
Typically metal sulfates are prepared by treating metal oxides, metal carbonates, or the metal itself with sulfuric acid:[7]
- Zn + H
2SO
4 → ZnSO
4 + H
2 - Cu(OH)
2 + H
2SO
4 → CuSO
4 + 2 H
2O - CdCO
3 + H
2SO
4 → CdSO
4 + H
2O + CO
2
Although written with simple anhydrous formulas, these conversions generally are conducted in the presence of water. Consequently the product sulfates are hydrated, corresponding to zinc sulfate ZnSO
4 · 7H2O, copper(II) sulfate CuSO
4 · 5H2O, and cadmium sulfate CdSO
4 · H2O.
Some metal sulfides can be oxidized to give metal sulfates.
Properties
There are numerous examples of ionic sulfates, many of which are highly soluble in water. Exceptions include calcium sulfate, strontium sulfate, lead(II) sulfate, barium sulfate, silver sulfate, and mercury sulfate, which are poorly soluble. Radium sulfate is the most insoluble sulfate known. The barium derivative is useful in the gravimetric analysis of sulfate: if one adds a solution of most barium salts, for instance barium chloride, to a solution containing sulfate ions, barium sulfate will precipitate out of solution as a whitish powder. This is a common laboratory test to determine if sulfate anions are present.
The sulfate ion can act as a ligand attaching either by one oxygen (monodentate) or by two oxygens as either a chelate or a bridge.[7] An example is the complex Co(en)
2(SO
4)]+
Br−
[7] or the neutral metal complex PtSO
4(PPh
3)
2] where the sulfate ion is acting as a bidentate ligand. The metal–oxygen bonds in sulfate complexes can have significant covalent character.
Uses and occurrence
Commercial applications
Sulfates are widely used industrially. Major compounds include:
- Gypsum, the natural mineral form of hydrated calcium sulfate, is used to produce plaster. About 100 million tonnes per year are used by the construction industry.
- Copper sulfate, a common algaecide, the more stable form (CuSO
4) is used for galvanic cells as electrolyte - Iron(II) sulfate, a common form of iron in mineral supplements for humans, animals, and soil for plants
- Magnesium sulfate (commonly known as Epsom salts), used in therapeutic baths
- Lead(II) sulfate, produced on both plates during the discharge of a lead–acid battery
- Sodium laureth sulfate, or SLES, a common detergent in shampoo formulations
- Polyhalite, K
2Ca
2Mg(SO
4)
4 · 2H2O, used as fertiliser.
Occurrence in nature
Sulfate-reducing bacteria, some anaerobic microorganisms, such as those living in sediment or near deep sea thermal vents, use the reduction of sulfates coupled with the oxidation of organic compounds or hydrogen as an energy source for chemosynthesis.
History
Some sulfates were known to alchemists. The vitriol salts, from the Latin vitreolum, glassy, were so-called because they were some of the first transparent crystals known.[8] Green vitriol is iron(II) sulfate heptahydrate, FeSO
4 · 7H2O; blue vitriol is copper(II) sulfate pentahydrate, CuSO
4 · 5H2O and white vitriol is zinc sulfate heptahydrate, ZnSO
4 · 7H2O. Alum, a double sulfate of potassium and aluminium with the formula K
2Al
2(SO
4)
4 · 24H2O, figured in the development of the chemical industry.
Environmental effects
Sulfates occur as microscopic particles (aerosols) resulting from fossil fuel and biomass combustion. They increase the acidity of the atmosphere and form acid rain. The anaerobic sulfate-reducing bacteria Desulfovibrio desulfuricans and D. vulgaris can remove the black sulfate crust that often tarnishes buildings.[9]
Main effects on climate

Reversal and accelerated warming
Hydrological cycle
Solar geoengineering
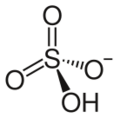
Hydrogensulfate (bisulfate)
| |
| Names | |
|---|---|
| IUPAC name
Hydrogensulfate[10]
| |
| Other names
Bisulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 2121 | |
PubChem CID
|
|
| |
| |
| Properties | |
| HSO− 4 | |
| Molar mass | 97.071 g/mol |
| Conjugate acid | Sulfuric acid |
| Conjugate base | Sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The hydrogensulfate ion (HSO−
4), also called the bisulfate ion, is the conjugate base of sulfuric acid (H
2SO
4).[11][lower-alpha 2] Sulfuric acid is classified as a strong acid; in aqueous solutions it ionizes completely to form hydronium (H
3O+
) and hydrogensulfate (HSO−
4) ions. In other words, the sulfuric acid behaves as a Brønsted–Lowry acid and is deprotonated to form hydrogensulfate ion. Hydrogensulfate has a valency of 1. An example of a salt containing the HSO−
4 ion is sodium bisulfate, NaHSO
4. In dilute solutions the hydrogensulfate ions also dissociate, forming more hydronium ions and sulfate ions (SO2−
4).
Other sulfur oxyanions
| Molecular formula | Name |
|---|---|
| SO2− 5 |
Peroxomonosulfate |
| SO2− 4 |
Sulfate |
| SO2− 3 |
Sulfite |
| S 2O2− 8 |
Peroxydisulfate |
| S 2O2− 7 |
Pyrosulfate |
| S 2O2− 6 |
Dithionate |
| S 2O2− 5 |
Metabisulfite |
| S 2O2− 4 |
Dithionite |
| S 2O2− 3 |
Thiosulfate |
| S 3O2− 6 |
Trithionate |
| S 4O2− 6 |
Tetrathionate |
See also
Notes
- ↑ Lewis assigned to sulfur a negative charge of two, starting from six own valence electrons and ending up with eight electrons shared with the oxygen atoms. In fact, sulfur donates two electrons to the oxygen atoms.
- ↑ The prefix "bi" in "bisulfate" comes from an outdated naming system and is based on the observation that there is twice as much sulfate (SO2−
4) in sodium bisulfate (NaHSO
4) and other bisulfates as in sodium sulfate (Na
2SO
4) and other sulfates. See also bicarbonate.
References
- ↑ Lewis, Gilbert N. (1916). "The Atom and the Molecule". J. Am. Chem. Soc. 38 (4): 762–785. doi:10.1021/ja02261a002. http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/papers/corr216.3-lewispub-19160400-18-large.html. (See page 778.)
- ↑ Pauling, Linus (1948). "The modern theory of valency". J. Chem. Soc. 17: 1461–1467. doi:10.1039/JR9480001461. PMID 18893624. https://authors.library.caltech.edu/59671/.
- ↑ Coulson, C. A. (1969). "d Electrons and Molecular Bonding". Nature 221 (5186): 1106. doi:10.1038/2211106a0. Bibcode: 1969Natur.221.1106C.
- ↑ Mitchell, K. A. R. (1969). "Use of outer d orbitals in bonding". Chem. Rev. 69 (2): 157. doi:10.1021/cr60258a001.
- ↑ 5.0 5.1 Cotton, F. Albert; Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry (2nd ed.). New York, NY: Wiley.
- ↑ 6.0 6.1 Stefan, Thorsten; Janoschek, Rudolf (Feb 2000). "How relevant are S=O and P=O Double Bonds for the Description of the Acid Molecules H2SO3, H2SO4, and H3PO4, respectively?". J. Mol. Modeling 6 (2): 282–288. doi:10.1007/PL00010730.
- ↑ 7.0 7.1 7.2 7.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Taylor, F. Sherwood (1942). Inorganic and Theoretical Chemistry (6th ed.). William Heinemann.
- ↑ Andrea Rinaldi (Nov 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the worlds cultural heritage". EMBO Reports 7 (11): 1075–1079. doi:10.1038/sj.embor.7400844. PMID 17077862.
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005, IUPAC, p. 129, http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005, IUPAC, p. 129, http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf
External links
 |