Chemistry:Lithium niobate

| |
 | |
| Names | |
|---|---|
| Other names
Lithium niobium oxide, lithium niobium trioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| LiNbO3 | |
| Molar mass | 147.846 g/mol |
| Appearance | colorless solid |
| Density | 4.30 g/cm3[1] |
| Melting point | 1,240 °C (2,260 °F; 1,510 K)[1] |
| None | |
| Band gap | 3.77 eV [2] |
Refractive index (nD)
|
no 2.3007, ne 2.2116[3] |
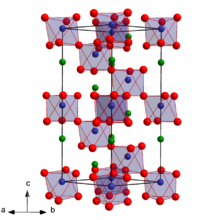
| Structure[4] | |
| Trigonal, hR30 | |
| R3c, No. 161 | |
| 3m (C3v) | |
a = 0.51501 nm, b = 0.51501 nm, c = 0.54952 nm α = 62.057°, β = 62.057°, γ = 60°
| |
Formula units (Z)
|
6 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
8 g/kg (oral, rat)[5] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium niobate (LiNbO
3) is a synthetic salt consisting of niobium, lithium, and oxygen. Its single crystals are an important material for optical waveguides, mobile phones, piezoelectric sensors, optical modulators and various other linear and non-linear optical applications.[6] Lithium niobate is sometimes referred to by the brand name linobate.[7]
Properties
Lithium niobate is a colorless solid, and it is insoluble in water. It has a trigonal crystal system, which lacks inversion symmetry and displays ferroelectricity, the Pockels effect, the piezoelectric effect, photoelasticity and nonlinear optical polarizability. Lithium niobate has negative uniaxial birefringence which depends slightly on the stoichiometry of the crystal and on temperature. It is transparent for wavelengths between 350 and 5200 nanometers.
Lithium niobate can be doped with magnesium oxide, which increases its resistance to optical damage (also known as photorefractive damage). Other available dopants are iron, zinc, hafnium, copper, gadolinium, erbium, yttrium, manganese and boron.
Growth
Single crystals of lithium niobate can be grown using the Czochralski process.[8]
After a crystal is grown, it is sliced into wafers of different orientation. Common orientations are Z-cut, X-cut, Y-cut, and cuts with rotated angles of the previous axes.[9]
Thin films
Thin-film lithium niobate (e.g. for optical wave guides) can be transferred to or grown on sapphire and other substrates, using the smart cut (ion slicing) process[10][11] or MOCVD process.[12] The technology is known as lithium niobate on insulator (LNOI).[13]
Nanoparticles
Nanoparticles of lithium niobate and niobium pentoxide can be produced at low temperature.[14] The complete protocol implies a LiH induced reduction of NbCl5 followed by in situ spontaneous oxidation into low-valence niobium nano-oxides. These niobium oxides are exposed to air atmosphere resulting in pure Nb2O5. Finally, the stable Nb2O5 is converted into lithium niobate LiNbO3 nanoparticles during the controlled hydrolysis of the LiH excess.[15] Spherical nanoparticles of lithium niobate with a diameter of approximately 10 nm can be prepared by impregnating a mesoporous silica matrix with a mixture of an aqueous solution of LiNO3 and NH4NbO(C2O4)2 followed by 10 min heating in an infrared furnace.[16]
Applications
Lithium niobate is used extensively in the telecommunications market, e.g. in mobile telephones and optical modulators.[17] Due to its large electro-mechanical coupling, it is the material of choice for surface acoustic wave devices. For some uses it can be replaced by lithium tantalate, LiTaO
3. Other uses are in laser frequency doubling, nonlinear optics, Pockels cells, optical parametric oscillators, Q-switching devices for lasers, other acousto-optic devices, optical switches for gigahertz frequencies, etc. It is an excellent material for manufacture of optical waveguides. It's also used in the making of optical spatial low-pass (anti-aliasing) filters.
In the past few years lithium niobate is finding applications as a kind of electrostatic tweezers, an approach known as optoelectronic tweezers as the effect requires light excitation to take place.[18][19] This effect allows for fine manipulation of micrometer-scale particles with high flexibility since the tweezing action is constrained to the illuminated area. The effect is based on the very high electric fields generated during light exposure (1–100 kV/cm) within the illuminated spot. These intense fields are also finding applications in biophysics and biotechnology, as they can influence living organisms in a variety of ways.[20] For example, iron-doped lithium niobate excited with visible light has been shown to produce cell death in tumoral cell cultures.[21]
Periodically poled lithium niobate (PPLN)
Periodically poled lithium niobate (PPLN) is a domain-engineered lithium niobate crystal, used mainly for achieving quasi-phase-matching in nonlinear optics. The ferroelectric domains point alternatively to the +c and the −c direction, with a period of typically between 5 and 35 µm. The shorter periods of this range are used for second-harmonic generation, while the longer ones for optical parametric oscillation. Periodic poling can be achieved by electrical poling with periodically structured electrode. Controlled heating of the crystal can be used to fine-tune phase matching in the medium due to a slight variation of the dispersion with temperature.
Periodic poling uses the largest value of lithium niobate's nonlinear tensor, d33 = 27 pm/V. Quasi-phase-matching gives maximum efficiencies that are 2/π (64%) of the full d33, about 17 pm/V.[22]
Other materials used for periodic poling are wide-band-gap inorganic crystals like KTP (resulting in periodically poled KTP, PPKTP), lithium tantalate, and some organic materials.
The periodic-poling technique can also be used to form surface nanostructures.[23][24]
However, due to its low photorefractive damage threshold, PPLN only finds limited applications, namely, at very low power levels. MgO-doped lithium niobate is fabricated by periodically poled method. Periodically poled MgO-doped lithium niobate (PPMgOLN) therefore expands the application to medium power level.
Sellmeier equations
The Sellmeier equations for the extraordinary index are used to find the poling period and approximate temperature for quasi-phase-matching. Jundt[25] gives
- [math]\displaystyle{ n^2_e \approx 5.35583 + 4.629 \times 10^{-7} f + \frac{0.100473 + 3.862 \times 10^{-8} f}{\lambda^2 - (0.20692 - 0.89 \times 10^{-8} f)^2} + \frac{100 + 2.657 \times 10^{-5} f}{\lambda^2 - 11.34927^2} - 1.5334 \times 10^{-2} \lambda^2, }[/math]
valid from 20 to 250 °C for wavelengths from 0.4 to 5 micrometers, whereas for longer wavelengths,[26]
- [math]\displaystyle{ n^2_e \approx 5.39121 + 4.968 \times 10^{-7} f + \frac{0.100473 + 3.862 \times 10^{-8} f}{\lambda^2 - (0.20692 - 0.89 \times 10^{-8} f)^2} + \frac{100 + 2.657 \times 10^{-5} f}{\lambda^2 - 11.34927^2} - (1.544 \times 10^{-2} + 9.62119 \times 10^{-10} \lambda) \lambda^2, }[/math]
which is valid for T = 25 to 180 °C, for wavelengths λ between 2.8 and 4.8 micrometers.
In these equations f = (T − 24.5)(T + 570.82), λ is in micrometers, and T is in °C.
More generally for ordinary and extraordinary index for MgO-doped LiNbO
3:
- [math]\displaystyle{ { n^2 \approx a_1 + b_1 f + \frac{a_2 + b_2 f}{\lambda^2 - (a_3 + b_3 f)^2} + \frac{a_4 + b_4 f}{\lambda^2 - a_5^2} - a_6 \lambda^2, } }[/math]
with:
| Parameters | 5% MgO-doped CLN | 1% MgO-doped SLN | |
|---|---|---|---|
| ne | no | ne | |
| a1 | 5.756 | 5.653 | 5.078 |
| a2 | 0.0983 | 0.1185 | 0.0964 |
| a3 | 0.2020 | 0.2091 | 0.2065 |
| a4 | 189.32 | 89.61 | 61.16 |
| a5 | 12.52 | 10.85 | 10.55 |
| a6 | 1.32×10−2 | 1.97×10−2 | 1.59×10−2 |
| b1 | 2.860×10−6 | 7.941×10−7 | 4.677×10−7 |
| b2 | 4.700×10−8 | 3.134×10−8 | 7.822×10−8 |
| b3 | 6.113×10−8 | −4.641×10−9 | −2.653×10−8 |
| b4 | 1.516×10−4 | −2.188×10−6 | 1.096×10−4 |
for congruent LiNbO
3 (CLN) and stochiometric LiNbO
3 (SLN).[27]
See also
- Crystal
- Crystal structure
- Crystallite
- Crystallization and engineering aspects
- Seed crystal
- Single crystal
- Laser-heated pedestal growth
- Micro-Pulling-Down
- Nickel niobate
References
- ↑ 1.0 1.1 Haynes, p. 4.70
- ↑ Zanatta, A.R. (August 2022). "The optical bandgap of lithium niobate (LiNbO3) and its dependence with temperature". Results Phys. 39: 105736–3pp. doi:10.1016/j.rinp.2022.105736.
- ↑ Haynes, p. 10.250
- ↑ Wilkinson, A. P.; Cheetham, A. K.; Jarman, R. H. (1993). "The defect structure of congruently melting lithium niobate". Journal of Applied Physics 74 (5): 3080–3083. doi:10.1063/1.354572. Bibcode: 1993JAP....74.3080W.
- ↑ "ChemIDplus – 12031-63-9 – PSVBHJWAIYBPRO-UHFFFAOYSA-N – Lithium niobate – Similar structures search, synonyms, formulas, resource links, and other chemical information". https://chem.nlm.nih.gov/chemidplus/rn/12031-63-9.
- ↑ Weis, R. S.; Gaylord, T. K. (1985). "Lithium Niobate: Summary of Physical Properties and Crystal Structure". Applied Physics A: Materials Science & Processing 37 (4): 191–203. doi:10.1007/BF00614817. Bibcode: 1985ApPhA..37..191W.
- ↑ Staebler, D.L.; Amodei, J.J. (1972). "Thermally fixed holograms in LiNbO3". Ferroelectrics 3 (1): 107–113. doi:10.1080/00150197208235297. Bibcode: 1972Fer.....3..107S., seen in Yeh, Pochi; Gu, Claire, eds (1995). Landmark Papers On Photorefractive Nonlinear Optics. World Scientific. p. 182. ISBN 9789814502979.
- ↑ Volk, Tatyana; Wohlecke, Manfred (2008). Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching. Springer. pp. 1–9. doi:10.1007/978-3-540-70766-0. ISBN 978-3-540-70765-3.
- ↑ Wong, K. K. (2002). Properties of Lithium Niobate. London, United Kingdom: INSPEC. pp. 8. ISBN 0-85296-799-3.
- ↑ Levy, M.; Osgood, R. M.; Liu, R.; Cross, L. E.; Cargill, G. S.; Kumar, A.; Bakhru, H. (1998-10-19). "Fabrication of single-crystal lithium niobate films by crystal ion slicing" (in en). Applied Physics Letters 73 (16): 2293–2295. doi:10.1063/1.121801. ISSN 0003-6951. Bibcode: 1998ApPhL..73.2293L. http://aip.scitation.org/doi/10.1063/1.121801.
- ↑ Lu, H.; Sadani, B.; Courjal, N.; Ulliac, G.; Smith, N.; Stenger, V.; Collet, M.; Baida, F. I. et al. (2012). "Enhanced electro-optical lithium niobate photonic crystal wire waveguide on a smart-cut thin film". Optics Express 20 (3): 2974–2981. doi:10.1364/oe.20.002974. PMID 22330535. https://opg.optica.org/oe/viewmedia.cfm?uri=oe-20-3-2974&html=true. Retrieved 2022-07-08.
- ↑ Feigelson, R. S. (1996). "Epitaxial growth of lithium niobate thin films by the solid source MOCVD method". Journal of Crystal Growth 166 (1–4): 1–16. doi:10.1016/0022-0248(95)00570-6. Bibcode: 1996JCrGr.166....1F.
- ↑ Hu, Hui; Yang, Jin; Gui, Li; Sohler, Wolfgang (2012). "Lithium niobate-on-insulator (LNOI): Status and perspectives". Silicon Photonics and Photonic Integrated Circuits III. 8431. pp. 84311D. doi:10.1117/12.922401. https://physik.uni-paderborn.de/fileadmin/physik/Alumni/Sohler/2012/SPIE_Photonics_Europe_Hu__LNOI_2012.pdf.
- ↑ Grange, R.; Choi, J.W.; Hsieh, C.L.; Pu, Y.; Magrez, A.; Smajda, R.; Forro, L.; Psaltis, D. (2009). "Lithium niobate nanowires: synthesis, optical properties and manipulation". Applied Physics Letters 95 (14): 143105. doi:10.1063/1.3236777. Bibcode: 2009ApPhL..95n3105G. http://link.aip.org/link/?APPLAB/95/143105/1.
- ↑ "New Synthesis of Nanosized Niobium Oxides and Lithium Niobate Particles and Their Characterization by XPS Analysis". Journal of Nanoscience and Nanotechnology 9 (8): 4780–4789. 2009. doi:10.1166/jnn.2009.1087. PMID 19928149.
- ↑ Grigas, A; Kaskel, S (2011). "Synthesis of LiNbO3 nanoparticles in a mesoporous matrix". Beilstein Journal of Nanotechnology 2: 28–33. doi:10.3762/bjnano.2.3. PMID 21977412.
- ↑ Toney, James (2015). Lithium Niobate Photonics. Artech House. ISBN 978-1-60807-923-0.
- ↑ Carrascosa, M.; García-Cabañes, A.; Jubera, M.; Ramiro, J. B.; Agulló-López, F. (2015). "LiNbO3: A photovoltaic substrate for massive parallel manipulation and patterning of nano-objects". Applied Physics Reviews (AIP Publishing) 2 (4): 040605. doi:10.1063/1.4929374. ISSN 1931-9401. Bibcode: 2015ApPRv...2d0605C.
- ↑ García-Cabañes, Angel; Blázquez-Castro, Alfonso; Arizmendi, Luis; Agulló-López, Fernando; Carrascosa, Mercedes (2018-01-30). "Recent Achievements on Photovoltaic Optoelectronic Tweezers Based on Lithium Niobate". Crystals (MDPI AG) 8 (2): 65. doi:10.3390/cryst8020065. ISSN 2073-4352.
- ↑ Blázquez-Castro, A.; García-Cabañes, A.; Carrascosa, M. (2018). "Biological applications of ferroelectric materials". Applied Physics Reviews (AIP Publishing) 5 (4): 041101. doi:10.1063/1.5044472. ISSN 1931-9401. Bibcode: 2018ApPRv...5d1101B.
- ↑ Blázquez-Castro, Alfonso; Stockert, Juan C.; López-Arias, Begoña; Juarranz, Angeles; Agulló-López, Fernando; García-Cabañes, Angel; Carrascosa, Mercedes (2011). "Tumour cell death induced by the bulk photovoltaic effect of LiNbO3:Fe under visible light irradiation". Photochemical & Photobiological Sciences (Springer Science and Business Media LLC) 10 (6): 956–963. doi:10.1039/c0pp00336k. ISSN 1474-905X. PMID 21336376.
- ↑ Meyn, J.-P.; Laue, C.; Knappe, R.; Wallenstein, R.; Fejer, M. M. (2001). "Fabrication of periodically poled lithium tantalate for UV generation with diode lasers". Applied Physics B 73 (2): 111–114. doi:10.1007/s003400100623. Bibcode: 2001ApPhB..73..111M.
- ↑ Grilli, Simonetta; Ferraro, Pietro; De Natale, Paolo; Tiribilli, Bruno; Vassalli, Massimo (2005). "Surface nanoscale periodic structures in congruent lithium niobate by domain reversal patterning and differential etching". Applied Physics Letters 87 (23): 233106. doi:10.1063/1.2137877. Bibcode: 2005ApPhL..87w3106G.
- ↑ Ferraro, P.; Grilli, S. (2006). "Modulating the thickness of the resist pattern for controlling size and depth of submicron reversed domains in lithium niobate". Applied Physics Letters 89 (13): 133111. doi:10.1063/1.2357928. Bibcode: 2006ApPhL..89m3111F.
- ↑ Jundt, Dieter H. (1997). "Temperature-dependent Sellmeier equation for the index of refraction [math]\displaystyle{ n_e }[/math] in congruent lithium niobate". Optics Letters 22 (20): 1553–1555. doi:10.1364/OL.22.001553. PMID 18188296. Bibcode: 1997OptL...22.1553J.
- ↑ Deng, L. H.; Gao, X. M.; Cao, Z. S.; Chen, W. D.; Yuan, Y.Q.; Zhang, W. J.; Gong, Z. B. (2006). "Improvement to Sellmeier equation for periodically poled LiNbO3 crystal using mid-infrared difference-frequency generation". Optics Communications 268 (1): 110–114. doi:10.1016/j.optcom.2006.06.082. Bibcode: 2006OptCo.268..110D.
- ↑ Gayer, O.; Sacks, Z.; Galun, E.; Arie, A. (2008). "Temperature and wavelength dependent refractive index equations for MgO-doped congruent and stoichiometric LiNbO3". Appl. Phys. B 91 (2): 343–348. doi:10.1007/s00340-008-2998-2. Bibcode: 2008ApPhB..91..343G.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External links
 |


