Chemistry:Deoxycytidine diphosphate
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
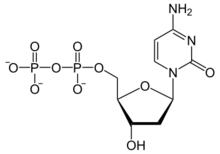
2′-Deoxycytidine 5′-(trihydrogen diphosphate)
| |
| Systematic IUPAC name
[(2R,3S,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxyoxolan-2-yl]methyl trihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | deoxycytidine+diphosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H15N3O10P2 | |
| Molar mass | 387.177 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Deoxycytidine diphosphate is a nucleoside diphosphate. It is related to the common nucleic acid CTP, or cytidine triphosphate, with the -OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy- part of the name), and with one fewer phosphoryl group than CTP .
2'-deoxycytidine diphosphate is abbreviated as dCDP.[1]
Synthesis of Cytidine Nucleotides
Deoxycytidine diphosphate is synthesized through the oxidation-reduction reaction of cytidine 5'-diphosphocholine which is catalyzed by the presence of ribonucleoside-diphosphate reductase.[2] Additionally, ribonucleoside-diphosphate reductase is capable of binding and catalyzing both the formation of deoxyribonucleotides from ribonucleotide.[3]
See also
References
- ↑ MeSH term, accessed Dec. 31, 2012
- ↑ Kandeel, Mahmoud; Al-Taher, Abdulla (2020-11-01). "Metabolic drug targets of the cytosine metabolism pathways in the dromedary camel (Camelus dromedarius) and blood parasite Trypanosoma evansi" (in en). Tropical Animal Health and Production 52 (6): 3337–3358. doi:10.1007/s11250-020-02366-8. ISSN 1573-7438. PMID 32926292. https://doi.org/10.1007/s11250-020-02366-8.
- ↑ Torrents, Eduard (2014). "Ribonucleotide reductases: essential enzymes for bacterial life". Frontiers in Cellular and Infection Microbiology 4: 52. doi:10.3389/fcimb.2014.00052. ISSN 2235-2988. PMID 24809024.
Further reading
- Kennedy, Eugene P.; Louise Fencil Borkenhagen; Sylvia Wagner Smith (1959). "Possible Metabolic Functions of Deoxycytidine Diphosphate Choline and Deoxycytidine Diphosphate Ethanolamine". Journal of Biological Chemistry 234 (8): 1998–2000. doi:10.1016/S0021-9258(18)69855-2. PMID 13673002. http://www.jbc.org/content/234/8/1998.full.pdf+html.
- Reichard, Peter (1962). "Enzymatic Synthesis of Deoxyribonucleotides: I. FORMATION OF DEOXYCYTIDINE DIPHOSPHATE FROM CYTIDINE DIPHOSPHATE WITH ENZYMES FROM ESCHERICHIA COLI". Journal of Biological Chemistry 237: 3513–3519. doi:10.1016/S0021-9258(19)70849-7. PMID 13973714. http://www.jbc.org/content/237/11/3513.full.pdf+html..
 |

