Chemistry:Cyclohexene
|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexene | |||
| Other names
Tetrahydrobenzene, 1,2,3,4-Tetrahydrobenzene, Benzenetetrahydride, Cyclohex-1-ene, Hexanaphthylene, UN 2256
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 906737 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 1659 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H10 | |||
| Molar mass | 82.143 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | sweet | ||
| Density | 0.8110 g/cm3 | ||
| Melting point | −103.5 °C (−154.3 °F; 169.7 K) | ||
| Boiling point | 82.98 °C (181.36 °F; 356.13 K) | ||
| slightly soluble in water | |||
| Solubility | miscible with organic solvents | ||
| Vapor pressure | 8.93 kPa (20 °C)
11.9 kPa (25 °C) | ||
Henry's law
constant (kH) |
0.022 mol·kg−1·bar−1 | ||
| -57.5·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4465 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |     
| ||
| GHS Signal word | Danger | ||
| H225, H302, H305, H311, H411 | |||
| P210, P233, P240, P241, P242, P243, P264, P270, P273, P280, P301+310, P301+312, P302+352, P303+361+353, P312, P322, P330, P331, P361, P363, P370+378, P391, P403+235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −12 °C (10 °F; 261 K) | ||
| 244 °C (471 °F; 517 K) | |||
| Explosive limits | 0.8–5 % | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1407 mg/kg (oral, rat) | ||
LCLo (lowest published)
|
13,196 ppm (mouse, 2 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 300 ppm (1015 mg/m3)[1] | ||
REL (Recommended)
|
TWA 300 ppm (1015 mg/m3)[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
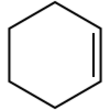
Cyclohexene is a hydrocarbon with the formula (CH
2)
4C
2H
2. It is a colorless liquid with a sharp odor. Although it is one of the simplest cycloalkene, it has few applications.
Production and uses
Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company.[3] The main product of the process is cyclohexane because cyclohexene is more easily hydrogenated than benzene.
In the laboratory, it can be prepared by dehydration of cyclohexanol.[4]
- C
6H
11OH → C
6H
10 + H
2O
Reactions and uses
Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene.[5] Cyclohexylbenzene is a precursor to both phenol and cyclohexanone.[6]
Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam.[7]
The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant in the presence of a tungsten catalyst.[8]
Bromination gives 1,2-dibromocyclohexane.[9]
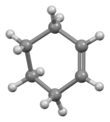
Structure
Cyclohexene is most stable in a half-chair conformation,[10] unlike the preference for a chair form of cyclohexane. One basis for the cyclohexane conformational preference for a chair is that it allows each bond of the ring to adopt a staggered conformation. For cyclohexene, however, the alkene is planar, equivalent to an eclipsed conformation at that bond.
See also
- Diels-Alder reaction
- Cyclohexa-1,3-diene
- Cyclohexa-1,4-diene
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0167". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0167.html.
- ↑ "Cyclohexene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/110838.html.
- ↑ Narisawa, Naoki & Katsutoshi Tanaka, "Cyclohexanol, method for producing cyclohexanol, and method for producing adipic acid", US patent 9771313, published 26 Sep 2017
- ↑ G. H. Coleman, H. F. Johnstone (1925). "Cyclohexene". Organic Syntheses 5: 33. doi:10.15227/orgsyn.005.0033.
- ↑ B. B. Corson, V. N. Ipatieff (1939). "Cyclohexylbenzene". Organic Syntheses 19: 36. doi:10.15227/orgsyn.019.0036.
- ↑ Plotkin, Jeffrey S. (2016-03-21). "What's New in Phenol Production?". American Chemical Society. https://www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/what-s-new-in-phenol-production-.html.
- ↑ Musser, Michael T. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_217.
- ↑ Reed, Scott M.; Hutchison, James E. (2000). "Green Chemistry in the Organic Teaching Laboratory: An Environmentally Benign Synthesis of Adipic Acid". J. Chem. Educ. 77 (12): 1627–1629. doi:10.1021/ed077p1627. Bibcode: 2000JChEd..77.1627R.
- ↑ H. R. Snyder, L. A. Brooks (1932). "1,2-Dibromocyclohexane". Organic Syntheses 12: 26. doi:10.15227/orgsyn.012.0026.
- ↑ Jensen, Frederick R.; Bushweller, C. Hackett (1969). "Conformational preferences and interconversion barriers in cyclohexene and derivatives". J. Am. Chem. Soc. 91 (21): 5774–5782. doi:10.1021/ja01049a013.
External links
- International Chemical Safety Card 1054
- NIOSH Pocket Guide to Chemical Hazards. "#0167". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0167.html.
- Material Safety Data Sheet for cyclohexene
- Safety MSDS data
- Reaction of Cyclohexene with Bromine and Potassium Permanganate
- Cyclohexene synthesis
- Data sheet at inchem.org
 |