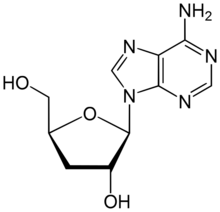
Chemistry:Cordycepin
| |
| Names | |
|---|---|
| IUPAC name
3′-Deoxyadenosine
| |
| Systematic IUPAC name
(2S,3R,5S)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolan-3-ol | |
| Other names
Cordycepine
9-(3-Deoxy-β-D-ribofuranosyl)adenine 3-dA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H13N5O3 | |
| Molar mass | 251.246 g·mol−1 |
| Melting point | 225.5 °C (437.9 °F; 498.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cordycepin, or 3'-deoxyadenosine, is a derivative of the nucleoside adenosine, differing from the latter by the replacement of the hydroxy group in the 3' position with a hydrogen. It was initially extracted from the fungus Cordyceps militaris,[1] but can now be produced synthetically.[2] It is also found in other Cordyceps species as well as Ophiocordyceps sinensis.[3]
Cordycepin is produced in cordyceps as a means of infecting insect populations, due to cordycepin's biological activity[4]
Because cordycepin is similar to adenosine, some enzymes cannot discriminate between the two.[citation needed] It can therefore participate in certain biochemical reactions (for example, 3-dA can trigger the premature termination of mRNA synthesis).[5][6] By acting as an adenosine analog, cordycepin was found to be the most potent molecular circadian clock resetter out of several screened compounds.[7]
Cordycepin has displayed cytotoxicity against some leukemic cell lines in vitro.[8][9][10] Additionally, cordycepin has been shown to display an effect in some types of other cancers, such as lung,[11] renal,[12] colon,[13] and breast cancer.[14] Cordycepin has been shown to reduce viable A549 lung cancer cell populations by 50%.[11]
Cordycepin has been found to produce rapid, robust imipramine-like antidepressant effects in animal models of depression, and these effects, similarly to those of imipramine, are dependent on enhancement of AMPA receptor signaling.[15]
Cordycepin has been shown to have anti-inflammatory qualities,[16] as well as the ability to defend against injury from cerebral ischemia in mice.[17]
See also
References
- ↑ Cunningham, K. G.; Manson, W.; Spring, F. S.; Hutchinson, S. A. (1950). "Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link.". Nature 166 (4231): 949. doi:10.1038/166949a0. PMID 14796634. Bibcode: 1950Natur.166..949C.
- ↑ Huang, Shen; Liu, Hui; Sun, Yanhua; Chen, Jian; Li, Xiufang; Xu, Jiangfeng; Hu, Yuwei; Li, Yuqing et al. (2018-01-01). "An effective and convenient synthesis of cordycepin from adenosine" (in en). Chemical Papers 72 (1): 149–160. doi:10.1007/s11696-017-0266-9. ISSN 1336-9075. https://doi.org/10.1007/s11696-017-0266-9.
- ↑ Zhou, X; Luo, L; Dressel, W; Shadier, G; Krumbiegel, D; Schmidtke, P; Zepp, F; Meyer, CU (2008). "Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis.". The American Journal of Chinese Medicine 36 (5): 967–80. doi:10.1142/S0192415X08006387. PMID 19051361.
- ↑ Raethong, Nachon; Wang, Hao; Nielsen, Jens; Vongsangnak, Wanwipa (2020). "Optimizing cultivation of Cordyceps militaris for fast growth and cordycepin overproduction using rational design of synthetic media" (in en). Computational and Structural Biotechnology Journal 18: 1–8. doi:10.1016/j.csbj.2019.11.003. PMID 31890138.
- ↑ Siev, M.; Weinberg, R.; Penman, S. (1969). "The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin". J. Cell Biol. 41 (2): 510–520. doi:10.1083/jcb.41.2.510. PMID 5783871.
- ↑ "Inhibition of polyadenylation reduces inflammatory gene induction". RNA 18 (12): 2236–50. 2012. doi:10.1261/rna.032391.112. PMID 23118416.
- ↑ Ju, Dapeng; Zhang, Wei; Yan, Jiawei; Zhao, Haijiao; Li, Wei; Wang, Jiawen; Liao, Meimei; Xu, Zhancong et al. (6 May 2020). "Chemical perturbations reveal that RUVBL2 regulates the circadian phase in mammals". Science Translational Medicine 12 (542): eaba0769. doi:10.1126/scitranslmed.aba0769. PMID 32376767.
- ↑ National Cancer Institute (2011-02-02). "Definition of cordycepin". http://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=42667. Retrieved 21 December 2015.
- ↑ Kodama, E.M.; McCaffrey, R.P.; Yusa, K.; Mitsuya, H. (February 2000). "Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells". Biochemical Pharmacology 59 (3): 273–281. doi:10.1016/S0006-2952(99)00325-1. PMID 10609556.
- ↑ Chou, S.M.; Lai, W.J.; Hong, T.W.; Lai, J.Y.; Tsai, S.H.; Chen, Y.H.; Yu, S.H.; Kao, C.H. et al. (October 2014). "Synergistic property of cordycepin in cultivated Cordyceps militaris-mediated apoptosis in human leukemia cells". Phytomedicine 21 (12): 1516–1524. doi:10.1016/j.phymed.2014.07.014. PMID 25442260.
- ↑ 11.0 11.1 Tuli, Hardeep Singh; Kumar, Gaurav; Sandhu, Sardul Singh; Sharma, Anil Kumar; Kashyap, Dharmbir (2015). "Apoptotic effect of cordycepin on A549 human lung cancer cell line". Turkish Journal of Biology 39: 306–311. doi:10.3906/biy-1408-14. https://journals.tubitak.gov.tr/biology/vol39/iss2/16.
- ↑ Hwang, In-Hu; Oh, Seung Yoon; Jang, Hyun-Jin; Jo, Eunbi; Joo, Jong Cheon; Lee, Kyung-Bok; Yoo, Hwa-Seung; Lee, Mi Young et al. (2017-10-18). Ahmad, Aamir. ed. "Cordycepin promotes apoptosis in renal carcinoma cells by activating the MKK7-JNK signaling pathway through inhibition of c-FLIPL expression" (in en). PLOS ONE 12 (10): e0186489. doi:10.1371/journal.pone.0186489. ISSN 1932-6203. PMID 29045468. Bibcode: 2017PLoSO..1286489H.
- ↑ Lee, Seung Yuan; Debnath, Trishna; Kim, Si-Kwan; Lim, Beong Ou (October 2013). "Anti-cancer effect and apoptosis induction of cordycepin through DR3 pathway in the human colonic cancer cell HT-29" (in en). Food and Chemical Toxicology 60: 439–447. doi:10.1016/j.fct.2013.07.068. PMID 23941773. https://linkinghub.elsevier.com/retrieve/pii/S0278691513005231.
- ↑ Lee, Dahae; Lee, Won-Yung; Jung, Kiwon; Kwon, Yong; Kim, Daeyoung; Hwang, Gwi; Kim, Chang-Eop; Lee, Sullim et al. (2019-08-26). "The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis" (in en). Biomolecules 9 (9): 414. doi:10.3390/biom9090414. ISSN 2218-273X. PMID 31454995.
- ↑ Li, Bai; Hou, Yangyang; Zhu, Ming; Bao, Hongkun; Nie, Jun; Zhang, Grace Y.; Shan, Liping; Yao, Yao et al. (2016). "3'-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway". International Journal of Neuropsychopharmacology 19 (4): pyv112. doi:10.1093/ijnp/pyv112. ISSN 1461-1457. PMID 26443809.
- ↑ Tan, Lu; Song, Xiaominting; Ren, Yali; Wang, Miao; Guo, Chuanjie; Guo, Dale; Gu, Yucheng; Li, Yuzhi et al. (March 2021). "Anti-inflammatory effects of cordycepin: A review" (in en). Phytotherapy Research 35 (3): 1284–1297. doi:10.1002/ptr.6890. ISSN 0951-418X. PMID 33090621. https://onlinelibrary.wiley.com/doi/10.1002/ptr.6890.
- ↑ Cheng, Zhenyong; He, Wei; Zhou, Xiaoxia; Lv, Qing; Xu, Xulin; Yang, Shanshan; Zhao, Chenming; Guo, Lianjun (2011-08-16). "Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro" (in en). European Journal of Pharmacology 664 (1): 20–28. doi:10.1016/j.ejphar.2011.04.052. ISSN 0014-2999. PMID 21554870. https://www.sciencedirect.com/science/article/pii/S0014299911004717.
 |

