Chemistry:Chromium(III) fluoride
| |

| |
| Names | |
|---|---|
| IUPAC name
Chromium(III) fluoride
| |
| Other names
Chromium trifluoride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| CrF 3 | |
| Molar mass |
|
| Appearance | green crystalline solid |
| Density | 3.8 g/cm3 (anhydrous) 2.2 g/cm3 (trihydrate) |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) (sublimes) |
| negligible (anhydrous) sparingly soluble (trihydrate) | |
| Solubility | Insoluble in alcohols Soluble in HF, HCl |
| +4370.0·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg/kg (guinea pig, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[1] |
REL (Recommended)
|
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger)
|
250 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium(III) fluoride is an inorganic compound with the chemical formula CrF
3. It forms several hydrates. The compound CrF
3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H
2O)
6]F
3 (violet) and [Cr(H
2O)
6]F
3 · 3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.[3]
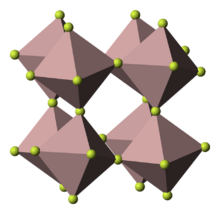
Structures
Like almost all compounds of chromium(III), these compounds feature octahedral Cr centres. In the anhydrous form, the six coordination sites are occupied by fluoride ligands that bridge to adjacent Cr centres. In the hydrates, some or all of the fluoride ligands are replaced by water.[4]
Production
Chromium(III) fluoride is produced from the reaction of chromium(III) oxide and hydrofluoric acid:[5]
- Cr
2O
3 + 6 HF + 9 H
2O → 2 [Cr(H
2O)
6]F
3
The anhydrous form is produced from hydrogen fluoride and chromic chloride:[6]
- CrCl
3 + 3 HF → CrF
3 + 3 HCl
Another method of synthesis of CrF
3 involves thermal decomposition of [NH
4]
3[CrF
6] (ammonium hexafluorochromate(III)):
- [NH
4]
3[CrF
6] → CrF
3 + 3 NH
3 + 3 HF
A mixed valence compound Cr
2F
5 (chromium(II,III) fluoride) is also known.[7]
Uses
Chromium(III) fluoride finds some applications as a mordant in textiles and as a corrosion inhibitor. Chromium(III) fluoride catalyzes the fluorination of chlorocarbons by HF.[8][9]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0141.html.
- ↑ "Chromium(III) compounds [as Cr(III)"]. Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/cr3m3.html.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ F.H. Herbstein, M. Kapon and G.M. Reisner, "Crystal structures of chromium(III) fluoride trihydrate. Structural chemistry of hydrated transition metal fluorides. Thermal decomposition of chromium(III) fluoride nonhydrate" Zeitschrift für Kristallographie 1985, volume 171, pp. 209
- ↑ Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a07_067
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN:0-7506-3365-4.
- ↑ Sturm. B.J. Phase Equilibria in the System Chromium(II)Fluoride-Chromium(III) Fluoride. Inorg. Chem., 1962, 1 (3), pp 665–672
- ↑ Mallikarjuna R. V. N.; Subramanian M. A. Fluoroolefin Manufacturing U.S. Patent 6,031,14, August 6, 1998; n.a.
- ↑ Ruh R. P.; Davis R. A. Proceess for Fluorinating Aliphatic Halohydrocarbons with a Chromium Fluoride catalyst and process for preparing the catalyst. U.S. Patent 2,745,886, May 15, 1956; n.a.
 |

