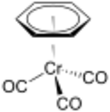
Chemistry:(Benzene)chromium tricarbonyl
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(benzene)tricarbonylchromium
| |||
| Other names
benzene tricarbonyl chromium, (benzene)chromium tricarbonyl, Benchrotrene, pi-benzenetricarbonylchromium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| Cr(C6H6)(CO)3 | |||
| Molar mass | 214.14 g/mol | ||
| Appearance | solid yellow crystals | ||
| Melting point | 163 to 166 °C (325 to 331 °F; 436 to 439 K) | ||
| nonsoluble | |||
| Solubility | THF, ether, benzene | ||
| Structure | |||
| tetrahedral, "piano stool" | |||
| Hazards | |||
| Main hazards | Harmful through inhalation, contact with skin, or swallowed | ||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H302, H312, H332 | |||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
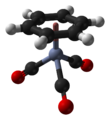
(Benzene)chromium tricarbonyl is an organometallic compound with the formula Cr(C6H6)(CO)3. This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as “piano stool” because of the planar arrangement of the aryl group and the presence of three CO ligands as "legs" on the chromium-bond axis.[1]
Preparation
(Benzene)tricarbonylchromium was first reported in 1957 by Fischer and Öfele, who prepared the compound by the carbonylation of bis(benzene)chromium.[2] They obtained mainly chromium carbonyl (Cr(CO)6) and traces of Cr(C6H6)(CO)3. The synthesis was optimized through the reaction of Cr(CO)6 and Cr(C6H6)2. For commercial purposes, a reaction of Cr(CO)6 and benzene is used:
- Cr(CO)6 + C6H6 → Cr(C6H6)(CO)3 + 3 CO
Applications
Complexes of the type (Arene)Cr(CO)3 have been well investigated as reagents in organic synthesis..[3] The aromatic ring of (benzene)tricarbonylchromium is substantially more electrophilic than benzene itself, allowing it to undergo nucleophilic addition reactions.[4]
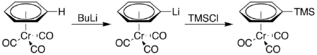
It is also more acidic, undergoing lithiation upon treatment with n-butyllithium. The resulting organolithium compound can then be used as a nucleophile in various reactions, for example, with trimethylsilyl chloride:
(Benzene)tricarbonylchromium is a useful catalyst for the hydrogenation of 1,3-dienes. The product alkene results from 1,4-addition of hydrogen. The complex does not hydrogenate isolated double bonds.
References
- ↑ Gilbert T. M. Bauer C. B., Rogers R. D. (1996). "Structures of (η6-benzene dimethylacetal)- and (η6-benzene diethylacetal)chromium tricarbonyl: structural evidence for the near-electroneutrality of the dialkylacetal substituent". Journal of Chemical Crystallography 26 (5): 355. doi:10.1007/BF01677100.
- ↑ Fischer, Ernst Otto; Őfele, Karl. (1957). “Über Aromatenkomplexe von Metallen, XIII Benzol-Chrom-Tricarbonyl,” Chemische Berichte, 90, 2532-5. doi:10.1002/cber.19570901117.
- ↑ E. Peter Kündig (2004). "Synthesis of Transition Metal η6-Arene Complexes". Topics Organomet Chem.. Topics in Organometallic Chemistry 7: 3–20. doi:10.1007/b94489. ISBN 978-3-540-01604-5.
- ↑ Herndon, James W; Laurent, Stéphane E. (2008). “(η6-Benzene)tricarbonylchromium,” in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Chichester, 2008. doi:10.1002/047084289X.rb025.pub2. Article Online Posting Date: March 15, 2009
 |