Chemistry:Chlorine trifluoride oxide
| |

| |
| Names | |
|---|---|
| IUPAC name
trifluoro(oxo)-λ5-chlorane
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| ClF3O | |
| Molar mass | 108.44 g·mol−1 |
| Density | 1.865 |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 29 °C (84 °F; 302 K) |
| Structure | |
| monoclinic | |
| C2/m | |
a = 9.826, b = 12.295, c = 4.901 α = 90°, β = 90.338°, γ = 90°[2]
| |
Lattice volume (V)
|
592.1 |
Formula units (Z)
|
8 |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| Related compounds | |
Related compounds
|
BrOF3; IOF3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.
Production
Chlorine oxide trifluoride was originally made at Rocketdyne[3] by treating dichlorine monoxide with fluorine. Other substances that could react with fluorine to make it includes sodium chlorite (NaClO2), and chlorine nitrate (ClONO2). The first published production method was a reaction of dichlorine monoxide with oxygen difluoride (OF2). Yet other production methods are reactions between ClO2F or ClO3F and chlorine fluorides.[4] A safer approach is the use chlorine nitrate with fluorine.
Reactions
As a Lewis base it can lose a fluoride ion to Lewis acids, yielding the difluorooxychloronium(V) cation (ClOF2+).[5] Compounds with this include: ClOF2BF4, ClOF2PF6, ClOF2AsF6, ClOF2SbF6, ClOF2BiF6, ClOF2VF6, ClOF2NbF6, ClOF2TaF6, ClOF2UF6, ClOF2, (ClOF2)2SiF6, ClOF2MoOF5, ClOF2Mo2O4F9,[4] ClOF2PtF6.[6]
Functioning as a Lewis acid, it can gain a fluoride ion from a strong base to yield a tetrafluorooxychlorate(V) anion: ClOF4− ion.[7] These include KClOF4, RbClOF4, and CsClOF4.[8] This allows purification of ClOF3, as at room temperature a solid complex is formed, but this decomposes between 50 and 70 °C. Other likely impurities either will not react with alkali fluoride, or if they do will not easily decompose.[3]
Chlorine trifluoride oxide fluoridates various materials such as chlorine monoxide, chlorine, glass or quartz.[3] ClOF3 + Cl2O → 2ClF + ClO2F;[6] 2ClOF3 + 2Cl2 → 6ClF + O2 at 200 °C[6]
Chlorine trifluoride oxide adds to chlorine fluorosulfate, ClOF3 + 2ClOSO2F → S2O5F2 + FClO2 + 2ClF. The reaction also produces SO2F2.[3]
Chlorine trifluoride oxide can fluoridate and add oxygen in the same reaction, reacting with molybdenum pentafluoride, silicon tetrafluoride, tetrafluorohydrazine (over 100 °C), HNF2, and F2NCOF. From HNF2 the main result was NF3O. From MoF5, the results were MoF6 and MoOF4.[3]
It reacts explosively with hydrocarbons.[3] With small amounts of water, ClO2F is formed along with HF.[3]
Over 280 °C ClOF3 decomposes to oxygen and chlorine trifluoride.[3]
Properties
The boiling point of chlorine trifluoride oxide is 29 °C.[9]
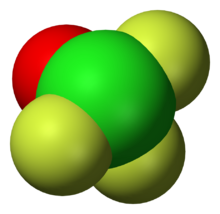
The shape of the molecule is a trigonal bipyramid, with two fluorine atoms at the top and bottom (apex) (Fa) and an electron pair, oxygen and fluorine (Fe) on the equator.[7] The Cl=O bond length is 1.405 Å, Cl-Fe 1.603 Å, other Cl-Fa 1.713 Å, ∠FeClO=109° ∠FaClO=95°, ∠FaClFe=88°. The molecule is polarised, Cl has a +1.76 charge, O has −0.53, equatorial F has −0.31 and apex F has −0.46. The total dipole moment is 1.74 D.[10]
References
- ↑ Urben, Peter (2017) (in en). Bretherick's Handbook of Reactive Chemical Hazards. Elsevier. p. 784. ISBN 9780081010594. https://books.google.com/books?id=rnXUDAAAQBAJ&pg=PA784.
- ↑ Ellern, Arkady; Boatz, Jerry A.; Christe, Karl O.; Drews, Thomas; Seppelt, Konrad (September 2002). "The Crystal Structures of ClF3O, BrF3O, and [NO]+[BrF4O]–". Zeitschrift für anorganische und allgemeine Chemie 628 (9–10): 1991–1999. doi:10.1002/1521-3749(200209)628:9/10<1991::AID-ZAAC1991>3.0.CO;2-1.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 (in en) Advances in Inorganic Chemistry and Radiochemistry. Academic Press. 1976. pp. 331–333. ISBN 9780080578675. https://books.google.com/books?id=EWlBFTxYth4C&pg=PA331.
- ↑ 4.0 4.1 Holloway, John H.; Laycock, David (1983) (in en). Advances in Inorganic Chemistry. Academic Press. pp. 178–179. ISBN 9780080578767. https://books.google.com/books?id=W4RngLN275wC&pg=PA178.
- ↑ Christe, Karl O.; Curtis, E. C.; Schack, Carl J. (September 1972). "Chlorine trifluoride oxide. VII. Difluorooxychloronium(V) cation, ClF2O+. Vibrational spectrum and force constants". Inorganic Chemistry 11 (9): 2212–2215. doi:10.1021/ic50115a046.
- ↑ 6.0 6.1 6.2 Schack, Carl J.; Lindahl, C. B.; Pilipovich, Donald.; Christe, Karl O. (September 1972). "Chlorine trifluoride oxide. IV. Reaction chemistry". Inorganic Chemistry 11 (9): 2201–2205. doi:10.1021/ic50115a043.
- ↑ 7.0 7.1 Christe, K.O.; Schack, C.J. (1976). Chlorine Oxyfluorides. Advances in Inorganic Chemistry and Radiochemistry. 18. pp. 319–398. doi:10.1016/S0065-2792(08)60033-3. ISBN 9780120236183.
- ↑ Christe, Karl O.; Schack, Carl J.; Pilipovich, Donald.; Christe, Karl O. (September 1972). "Chlorine trifluoride oxide. V. Complex formation with Lewis acids and bases". Inorganic Chemistry 11 (9): 2205–2208. doi:10.1021/ic50115a044.
- ↑ Pilipovich, Donald.; Lindahl, C. B.; Schack, Carl J.; Wilson, R. D.; Christe, Karl O. (September 1972). "Chlorine trifluoride oxide. I. Preparation and properties". Inorganic Chemistry 11 (9): 2189–2192. doi:10.1021/ic50115a040.
- ↑ Oberhammer, Heinz.; Christe, Karl O. (January 1982). "Gas-phase structure of chlorine trifluoride oxide, ClF3O". Inorganic Chemistry 21 (1): 273–275. doi:10.1021/ic00131a050.
 |

