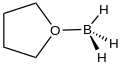
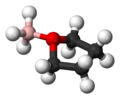
Chemistry:Borane–tetrahydrofuran
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H11BO | |
| Molar mass | 85.94 g·mol−1 |
| Appearance | White solid |
| Melting point | 66 °C (151 °F; 339 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H225, H260, H302, H315, H318, H319, H335 | |
| P210, P223, P231+232, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P335+334, P337+313 | |
| Flash point | −17 °C (1 °F; 256 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Borane–tetrahydrofuran is an adduct derived from borane and tetrahydrofuran (THF). These solutions, which are colorless, are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds. The use of borane–tetrahydrofuran has been displaced by borane–dimethylsulfide, which has a longer shelf life and effects similar transformations.[1]
Preparation and uses
The complex is commercially available but can also be generated by the dissolution of diborane in THF. Alternatively, it can be prepared by the oxidation of sodium borohydride with iodine in THF.[2]
The complex can reduce carboxylic acids to alcohols and is a common route for the reduction of amino acids to amino alcohols[3] (e.g. valinol). It adds across alkenes to give organoboron compounds that are useful intermediates.[4] The following organoboron reagents are prepared from borane-THF: 9-borabicyclo[3.3.1]nonane, Alpine borane, diisopinocampheylborane. It is also used as a source of borane (BH3) for the formation of adducts.[5]
Safety
The solution is highly sensitive to air, requiring the use of air-free techniques.[1]
See also
References
- ↑ 1.0 1.1 Marek Zaidlewicz, Herbert C. Brown, Santhosh F. Neelamkavil, "Borane–Tetrahydrofuran" Encyclopedia of Reagents for Organic Synthesis, 2008 John Wiley & Sons. doi:10.1002/047084289X.rb241.pub2
- ↑ Kanth, J. V. Bhaskar; Periasamy, Mariappan (1 September 1991). "Selective reduction of carboxylic acids into alcohols using sodium borohydride and iodine". The Journal of Organic Chemistry 56 (20): 5964–5965. doi:10.1021/jo00020a052.
- ↑ McKennon, Marc J.; Meyers, A. I.; Drauz, Karlheinz; Schwarm, Michael (June 1993). "A convenient reduction of amino acids and their derivatives". The Journal of Organic Chemistry 58 (13): 3568–3571. doi:10.1021/jo00065a020.
- ↑ Kabalka, George W.; Maddox, John T.; Shoup, Timothy; Bowers, Karla R. (1996). "A Simple And Convenient Method For The Oxidation Of Organoboranes Using Sodium Perborate: (+)-isopinocampheol". Organic Syntheses 73: 116. http://www.orgsyn.org/demo.aspx?prep=CV9P0522.
- ↑ Crépy, Karen V. L.; Imamoto, Tsuneo (2005). "Preparation Of (S,S)-1,2-Bis-(tert-butylmethylphosphino)ethane ((S,S)-t-Bu-BISP*) As A Rhodium Complex". Organic Syntheses 82: 22. http://www.orgsyn.org/demo.aspx?prep=v82p0022.
 |

