Chemistry:Diisopinocampheylborane
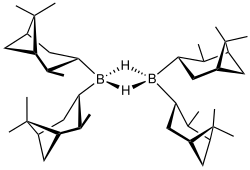 Structure of (+)-Diisopinocampheylborane dimer
| |
| Names | |
|---|---|
| IUPAC name
Di[(1S,2R,3S,5S)-pinan-3-yl]borane
| |
| Systematic IUPAC name
Bis[(1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-yl]borane | |
| Other names
(+)-Di-3-pinanylborane; Diisopinocampheylborane; Ipc2BH dimer
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | Ipc2BH |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H35B | |
| Molar mass | 286.31 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.044 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols. The reagent is often depicted as a monomer but like most hydroboranes, it is dimeric with B-H-B bridges.[1]
Preparation
Diisopinocampheylborane was originally prepared by hydroboration of excess α-pinene with borane,[2] but it is now more commonly generated from borane-methyl sulfide (BMS).[3]
The compound can be isolated as a solid, but because it is quite sensitive to water and air, it is often generated in situ and used as a solution. The synthesis is complicated by a number of factors, including the tendency of the compound to eliminate pinene.[1]
Diisopinocampheylborane is often represented as a monomer (including in this article), but X-ray crystallography establishes a dimeric structure.[1]
Reactions
Oxidation of diisopinocampheylborane with basic hydrogen peroxide gives isopincampheol. Methanolysis gives methoxydiisopinocampheylborane
Hydroboration
Because of the large size of the α-pinenyl substituents, diisopinocampheylborane only hydroborates unhindered alkenes. These reactions proceed with high enantioselectivity. 2-Butene, 2-pentene, 3-hexene are converted to the respective chiral alcohols in high ee's.[4] Norbornene under the same conditions gave an 83% ee. Heterocycles (dihydrofuran, dihydrothiophene, dihydropyrrole, tetrahydropyran) give the alcohols in ≥99% ee; the high ee's reflect their constrained conformations.[5]
It adds to alkynes to form the corresponding vinyldiisopinocampheylboranes
In a highly stereoselective reaction, allyldiisopinocampheylboranes converts aldehydes to the homologated alcohols, rapidly even at -100 °C.[6] The alkyldiisopinocampheylboranes, which result from the addition to alkenes, usefully react with a range of different reagents. Hydroxylamine-O-sulfonic acid provides 3-pinanamine.[7]
Also useful is the reaction of diisopinocampheylborane with an aldehyde (RCHO) to give the chiral boronic ester, (isopinocampheyl)2BOCH2(R), which can be further used is a number of reactions e.g. Suzuki reaction.[4]
Related campheylboranes
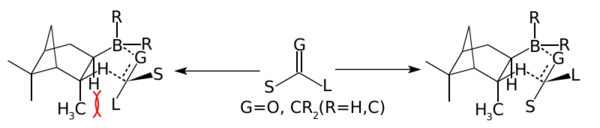
Treatment of diisopinocampheylborane with TMEDA give the crystalline adduct of monoisopinocampheylborane. This adduct reacts with boron trifluoride to liberate the monoisopinocampheylborane (IpcBH2) in 100% ee.[8] Monoisopinocampheylborane reacts with a variety of alkenes.[4] Two other reagents have been developed for the hydroboration of ketones:
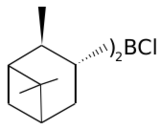
In the above mechanism where G=O and R is Ipc and Cl or 9-Borabicyclononane. Diisopinocampheylchloroborane (Ipc2BCl) is produced by treating diisopinocampheylborane with hydrogen chloride. The chloride is reported to be more stable that the trialkyl boranes,[4] it works well with aryl alkyl ketones and tert-butyl alkyl ketones. Diisopinocampheylchloroborane is often complementary with diisopinocampheylborane, where one provides the R enantiomer and the other the S, the enantioselectivity is typically very high.[9][10]
Alpine-borane is produced by hydroborating α-pinene with 9-borabicyclononane.[4] Both of these reagents can be improved upon by using 2-ethylapopinene in place of α-pinene, 2-ethylapopinene has an ethyl group in place of the methyl in α-pinene. The additional steric bulk improves the stereoselectivity of the reduction.
Diisopinocampheylborane reacts with methanol to give diisopinocampheylmethoxyborane, which in turn reacts with an allyl or crotyl Grignard reagent to give B-allyldiisopinocampheylborane. This can then undergo an asymmetric allylboration to give a chiral homologated alcohol, which is a useful building block in a chiral synthesis.
References
- ↑ 1.0 1.1 1.2 Fürstner, Alois; Bonnekessel, Melanie; Blank, Jarred T.; Radkowski, Karin; Seidel, Günter; Lacombe, Fabrice; Gabor, Barbara; Mynott, Richard (2007). "Total Synthesis of Myxovirescin A1". Chemistry – A European Journal 13 (31): 8762–8783. doi:10.1002/chem.200700926. PMID 17768720.
- ↑ Brown, Herbert C.; Yoon, Nung Min (1976). "Hydroboration. 44. Diisopinocampheylborane of High Optical Purity. Asymmetric Synthesis via Hydroboration with Essentially Complete Asymmetric Induction". Israel Journal of Chemistry 15 (1–2): 12–16. doi:10.1002/ijch.197600004. ISSN 0021-2148.
- ↑ C. F. Lane, J. J. Daniels (1972). "(−)-Isopincampheol". Organic Syntheses 52: 59. doi:10.15227/orgsyn.052.0059.
- ↑ 4.0 4.1 4.2 4.3 4.4 Brown, Herbert C.; George Zweifel (1961). "Hydroboration as a convenient procedure for the asymmetric synthesis of alcohols of high optical purity". J. Am. Chem. Soc. 83 (2): 486–487. doi:10.1021/ja01463a055.
- ↑ Brown, Herbert C.; Veeraraghavan Ramachandran (1991). "The boron approach to asymmertric synthesis". Pure Appl. Chem. 63 (3): 307–316. doi:10.1351/pac199163030307.
- ↑ Raj K. Dhar, Kanth V. B. Josyula, Robert Todd "Diisopinocampheylborane" in Encyclopedia of Reagents for Organic Synthesis, 2006, John Wiley & Sons, New York. doi:10.1002/047084289X.rd248.pub2. Article Online Posting Date: September 15, 2006
- ↑ Michael W. Rathke, Alan A. Millard. (1978). "Boranes in functionalization of olefins to amines: 3-Pinanamine". Organic Syntheses 58: 32. http://www.orgsyn.org/demo.aspx?prep=CV6P0943.; Collective Volume, 6, pp. 943
- ↑ Brown, Herbert C.; John R. Schwier; Bakthan Singaram (1978). "Simple synthesis of monoisopinocampheylborane of high optical purity". J. Org. Chem. 43 (32): 4395–4397. doi:10.1021/jo00416a042.
- ↑ Ramachandran, P.Veeraraghavan; Chen, Guang-Ming; Brown, Herbert C. (1996). "Efficient general asymmetric syntheses of 3-substituted 1(3H)-isobenzofuranones in very high enantiomeric excess". Tetrahedron Letters 37 (13): 2205–2208. doi:10.1016/0040-4039(96)00260-2. ISSN 0040-4039.
- ↑ Ramachandran, V. P.; S. Pitre; Herbert C. Brown (2002). "Selective Reductions. 59. Effective Intramolecular Asymmetric Reductions of α-,β and γ-Keto Acids with Diisopinocampheylborane and Intermolecular Asymmetric Reductions of the Corresponding Esters with B-Chlorodiisopinocampheylborane". J. Org. Chem. 67 (15): 5315–5319. doi:10.1021/jo025594y. PMID 12126421.
 |