Chemistry:Avanafil
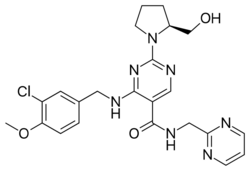 | |
 Avanafil is a PDE5 inhibitor | |
| Clinical data | |
|---|---|
| Trade names | Stendra |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614010 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C23H26ClN7O3 |
| Molar mass | 483.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Avanafil is a PDE5 inhibitor approved for erectile dysfunction by the FDA on April 27, 2012[2] and by EMA on June 21, 2013.[3] Avanafil is sold under the brand names Stendra and Spedra. It was invented at Mitsubishi Tanabe Pharma, formerly known as Tanabe Seiyaku Co.,[4] and licensed to Vivus Inc., which partnered with Menarini Group to commercialise Spedra in over forty European countries, Australia, and New Zealand.[5] Metuchen Pharmaceuticals obtained exclusive rights within the United States.[6]
Avanafil acts by inhibiting a specific phosphodiesterase type 5 enzyme found in various body tissues, primarily in the corpus cavernosum penis.[7] Other similar drugs are sildenafil, tadalafil and vardenafil. The advantage of avanafil is that it has very fast onset of action compared with other PDE5 inhibitors. It is absorbed quickly, reaching a maximum serum concentration in about thirty to forty-five minutes.[8] About two-thirds of the participants were able to engage in sexual activity within fifteen minutes.[8]
Medical use
Avanafil is used to treat erectile dysfunction (ED).[9]
Adverse effects
Although avanafil is generally well tolerated, dose dependent adverse effects can occur.[8] The most common adverse effects include headache, flushing, nasopharyngitis, nasal congestion, and back pain.[8] While it is also uncommon, there is a potential for visual disturbances to occur in patients.[8]
Mechanism of action
Avanafil inhibits phosphodiesterase-5, preventing the degradation of cGMP.[10][11] The increased levels of cGMP causes vasodilation, resulting in an increased blood flow in the penis.[11] Avanafil's mechanism of action takes places once nitric oxide is released, in association with sexual stimulation.[11]
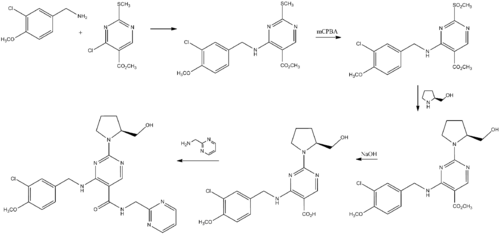
Synthesis
Avanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:[4]
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2016". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2016.
- ↑ "Stendra FDA Approval History". Drugs.com. https://www.drugs.com/history/stendra.html.
- ↑ "Spedra (avanafil)". European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002581/human_med_001661.jsp&mid=WC0b01ac058001d124.
- ↑ 4.0 4.1 Yamada K, Matsuki K, Omori K Kikkawa K, "Aromatic nitrogen-containing 6-membered cyclic compounds", US patent 6797709, issued 11 December 2003, assigned to Tanabe Seiyaku Co
- ↑ "VIVUS Announces Avanafil Partnership With Menarini". Vivus Inc. http://ir.vivus.com/releasedetail.cfm?releaseid=775706.
- ↑ "VIVUS and Metuchen Pharmaceuticals Announce License Agreement for Commercial Rights to Stendra". Vivus Inc. 3 October 2016. http://ir.vivus.com/news-releases/news-release-details/vivus-and-metuchen-pharmaceuticals-announce-license-agreement.
- ↑ "avanafil, Spedra". Medicine Net. http://www.medicinenet.com/avanafil_stendra/article.htm.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Avanafil for erectile dysfunction". The Annals of Pharmacotherapy (Sage Publishing) 47 (10): 1312–1320. October 2013. doi:10.1177/1060028013501989. PMID 24259695.
- ↑ "Avanafil for treatment of erectile dysfunction: review of its potential". Vascular Health and Risk Management 8: 517–523. 2012. doi:10.2147/VHRM.S26712. PMID 22973106.
- ↑ "Avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction". The Journal of Urology 188 (2): 668–674. August 2012. doi:10.1016/j.juro.2012.03.115. PMID 22704456.
- ↑ 11.0 11.1 11.2 "Avanafil: A Review of Its Use in Patients with Erectile Dysfunction". Drugs & Aging 30 (10): 853–62. October 2013. doi:10.1007/s40266-013-0112-x. PMID 23955441.
External links
- "Avanafil". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/avanafil.
 |