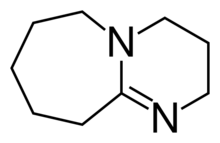
Chemistry:1,8-Diazabicyclo(5.4.0)undec-7-ene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3,4,6,7,8,9,10-Octahydropyrimido[1,2-a]azepine | |
| Other names
DBU, Diazabicycloundecene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H16N2 | |
| Molar mass | 152.241 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.018 g/mL liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 261 °C (502 °F; 534 K) (1 atm), 80 to 83 °C (0.6 mmHg) |
| ethers, alcohols | |
| Acidity (pKa) | 13.5±1.5[1] (pKa of conjugate acid in water); 24.34[2] (pKa of conjugate acid in acetonitrile) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H302, H312, H314, H412 | |
| P260, P264, P270, P273, P280, P301+310, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | 119.9 °C (247.8 °F; 393.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base.[3]
Occurrence
Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge Niphates digitalis.[4] The biosynthesis of DBU has been proposed to begin with adipaldehyde and 1,3-diaminopropane.

Uses
As a reagent in organic chemistry, DBU is used as a catalyst, a ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy resins and polyurethane.
It is used in the separation of fullerenes in conjunction with trimethylbenzene. It reacts with C70 and higher fullerenes, but not with C60.
It is useful for dehydrohalogenations.[5]
See also
References
- ↑ Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta 87 (4): 385–395. doi:10.5562/cca2472.
- ↑ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ↑ Ghosh, Nandita (2004). "DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) - A Nucleophillic Base". Synlett (3): 574–575. doi:10.1055/s-2004-815436.
- ↑ 4.0 4.1 E. L. Regalado; Judith Mendiola; Abilio Laguna; Clara Nogueiras; Olivier P Thomas (2010). "Polar alkaloids from the Caribbean marine sponge Niphates digitalis". Nat. Prod. Commun. 5 (8): 1187–1190. PMID 20839615.
- ↑ Savoca, Ann C.; Urgaonkar, Sameer (2006). "1,8-Diazabicyclo[5.4.0]undec-7-ene". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd011.pub2. ISBN 0-471-93623-5.
 |

